Abstract
Background
The best outcomes for treating childhood obesity have come from comprehensive family-based programmes. However there are questions over their generalisability.
Objective
To examine the acceptability and effectiveness of ‘family-based behavioural treatment’ (FBBT) for childhood obesity in an ethnically and socially diverse sample of families in a UK National Health Service (NHS) setting.
Methods
In this parallel group, randomised controlled trial, 72 obese children were randomised to FBBT or waiting list control. Primary outcomes were body mass index (BMI) and BMI standard deviation scores (SDS). Secondary outcomes were weight, weight SDS, height, height SDS, waist, waist SDS, fat mass index, fat free mass index, blood pressure, and psychosocial measures. Outcomes were assessed at baseline and post-treatment, with analyses on the 6 month data done on an intent-to-treat (ITT) basis. Follow-up anthropometric data were collected at twelve months for the treatment group.
Results
ITT analyses included all children with baseline data (n=60). There were significant BMI SDS changes (p<0.01) for the treatment and control groups of −0.11 (0.16) and −0.10 (1.6). The treatment group showed a significant reduction in systolic blood pressure (−0.24 (0.7), p<0.05) and improvements in quality of life and eating attitudes (p<0.05), with no significant changes for the control group. However the between-group treatment effects for BMI, body composition, blood pressure and psychosocial outcomes were not significant. There was no overall change in BMI or BMI SDS from 0-12 months for the treatment group. No adverse effects were reported.
Conclusions
Both treatment and control groups experienced significant reductions in level of overweight, but with no significant difference between them. There were no significant group differences for any of the secondary outcomes.
Keywords: paediatric obesity, behavioural treatment, randomised controlled trial
Introduction
Childhood obesity adversely affects children’s current and future health. In childhood, it increases the risk of hyperlipidaemia, hypertension, insulin resistance, abnormal glucose tolerance, asthma, and sleep apnoea among other conditions,1-2 and is negatively associated with quality of life and self-esteem.3 Childhood obesity also tracks into adulthood.4 Health Survey for England data show that 16.8% of boys and 15.2% of girls aged 2-15 years were obese, and 14.6% and 14.0% were overweight, in 2008.5 The potential health benefits for children who can reduce weight are substantial. Improvements in CVD risk profile have been reported in the short-term6 and long-term.7-9 Treatment programmes have also been shown to improve self-worth,10 body satisfaction and parent-reported child problems,11-12 and eating disorder symptoms.11
There continues to be sparse evidence on which treatments are effective, especially in healthcare settings. The best outcomes are from comprehensive family-based programmes; a recent meta-analysis of randomised controlled trials found a small but statistically significant effect on level of overweight (−0.06 BMI SDS) in children less than 12 years of age at 6 month follow-up, although the effect was no longer significant at 12 months.13 Particular shortcomings noted in this review were studies being under-powered, not reporting power calculations, and not carrying out intention-to-treat (ITT) analyses; the latter potentially resulting in an over-estimation of treatment effects. The most widely cited intervention is ‘Family-Based Behavioural Treatment’ (FBBT); a programme developed in the US that targets families with obese 8-12 year olds. Positive outcomes have been reported in both the short-term14-15 and the long-term.16-17 However, the generalisability of FBBT’s efficacy is uncertain because these studies have been carried out largely within a single academic setting. Few other studies have tested FBBT and none were RCTs; although all produced significant reductions in level of overweight.18-20 One had a comparison condition but failed to find a significant treatment effect.19 Our aim was to examine the acceptability and effectiveness of FBBT in an ethnically and socially diverse group of children in a UK National Health Service (NHS) setting. Given the existing strong evidence base, we delivered FBBT as described by Epstein and colleagues,21 with minimal adaptation. We piloted the programme with 33 families in 2002-2003; 27 families completed the treatment with a mean reduction in percent body mass index (% BMI) of 8.4, and a reduction of body mass index standard deviation score (BMI SDS) of 0.15 (p<0.001).22
The primary aim of the present study was to examine the impact of a 6 month FBBT programme carried out in a hospital setting on level of overweight (indexed by BMI SDS and BMI) in overweight and obese children aged 8-12 years, compared with a waiting list control group. We also examined effects on waist circumference, body composition, blood pressure, well-being (mood, self esteem, quality of life), and eating attitudes. The CONSORT statement format is used for reporting trial methods and outcomes.23-24
Patients and Methods
Design
Key stages for developing and evaluating complex health interventions are proposed in a framework by the UK Medical Research Council.25. This randomised controlled trial (RCT) forms part of the evaluation phase of the framework following the feasibility/pilot stage. Families were randomly allocated, in equal numbers, to an intervention group or a 6 month waiting-list control group. Families were recruited and randomised in five waves, each with approximately 15 children. Randomisation was carried out by a statistician; each child was given an ID code, and computer-generated random numbers were used to allocate them to a treatment condition. Families were informed of their allocation and commenced treatment or entered the waiting list period (with no input provided from the study team); treatment began within one month of randomisation. It was not possible to blind families or clinicians to treatment allocation because of the nature of the intervention, but the researcher collecting anthropometric data was blinded to group allocation unless families disclosed this information.
Participants
The study took place at Great Ormond Street Hospital for children (GOSH), in London between June 2004 and January 2008. Children were eligible to participate if they were 8-12 years of age, overweight or obese according to the International Obesity Task Force (IOTF) definition,26 had at least one parent or guardian willing to participate in treatment, and parent and child had sufficient command of English to participate in groups and understand the programme materials. Exclusion criteria were an identified medical cause for obesity (e.g. hypothyroidism, Prada Willi syndrome, single gene defects), type 2 diabetes, taking obesity medication, undergoing obesity treatment, significant learning difficulties, significant mental health problems in child or parent, or currently receiving psychological or psychiatric treatment including psychotrophic medication.
Participants were recruited through local professional networks in primary and secondary care, from schools and through information in local media. Families responding through the media were asked to seek a referral from their general practitioner (GP) to ensure that the intervention was properly integrated with their health care. Referred children were invited to an assessment appointment with one of the study clinicians, and an outpatient appointment with a paediatrician and a researcher who took anthropometric and body composition measures. The aims of the assessment were to provide the family with further information about the study, assess motivation and practicalities for attending (e.g. travel, care for other children), establish eligibility (as per the criteria outlined above), and collect baseline anthropometric, medical and psychometric data. The motivational assessment included children and parents’ independent ratings of motivation for making lifestyle changes as well perceived benefits of and barriers to change. Factors relating to the family or social context that could impact on ability to implement recommended changes were also discussed. Families in receipt of state benefits were reimbursed travel expenses for assessment appointments and treatment sessions, as per hospital policy.
Ethics
Ethical approval was obtained from the Research Ethics Committee at Great Ormond Street/Institute of Child Health (registration number 03BS18). Written informed consent was obtained from all children and parents/guardians.
Description of the intervention
FBBT is a structured intervention comprising advice on whole-family lifestyle change with a behavioural weight control programme for the overweight child. Children were required to attend with one parent or carer with a maximum of 8-10 families per group. The aims were to reduce fat and energy intake, increase physical activity, and change parent-child interactions. The components have been previously described21 and the programme was adapted for use with British families.22 Briefly, the behavioural programme is based on learning theory and uses behaviour modification techniques such as self-monitoring (daily food and activity diaries), goal setting, positive reinforcement, stimulus control, and relapse prevention to modify behaviours. Parents are instructed in behaviour management principles to support their child’s behaviour change and make changes to the home environment to encourage family-wide uptake of healthy lifestyle behaviours. Cognitive components of the programme include advice on managing teasing and general problem-solving.
The key dietary targets were i) to follow a regular eating pattern, ii) to reduce snacking to no more than two occasions per day, and iii) to consume a balanced diet (as described in the ‘eatwell plate’27 and the ‘Traffic Light’ system21) in appropriate quantities. Key physical activity targets were i) to reduce time spent in sedentary behaviours and ii) to increase the time spent in lifestyle or structured activity in line with the current UK recommendation of 60 minutes a day.28
Feedback from the pilot resulted in the addition of three maintenance sessions to the original 12 treatment sessions to reinforce longer-term behaviour change; the intervention therefore comprised 15 sessions over 6 months (10 weekly, 3 fortnightly, 2 monthly). Sessions took place in the late afternoon (after the school day) and lasted for approximately 1½ hours; each consisted of a brief review (5-10 minutes) with individual families where they were given feedback and weighed, followed by concurrent but separate parent and child group sessions as in Epstein’s protocol. The contents of the treatment and maintenance sessions are outlined in Supplemental Table 1. The parents’ groups were delivered by clinicians with experience of working with parents and families (psychologist, family therapist or experienced dietitian) and the children’s groups were delivered by a dietitian with experience of working with children and a researcher who assisted. Additional researchers carried out the brief one to-one family reviews.
Outcomes
The primary outcome measures were post-treatment BMI SDS and BMI. BMI has been proposed as a more sensitive measure of change in very overweight children,29 but because the majority of treatment studies report BMI SDS, we present both. Secondary anthropometric outcomes were post-treatment %BMI, weight, weight SDS, height, height SDS, waist, and waist SDS. Standard deviation scores for BMI, weight, height, and waist were calculated from raw values by adjusting for age and gender using British 1990 reference data.30-32 The LMSgrowth macro (http://homepage.mac.com/tjcole) was used. We included %BMI to allow for comparison with results reported by Epstein and colleagues,17 calculated using the percentage of the median BMI for the child’s age and gender. Weight was measured using Tanita electronic scales (model HD 352, Tanita) and height using a Harpenden stadiometer (Holtain, UK). Weights and heights were measured by trained personnel according to a standard protocol. All waist measurements were taken by a single researcher, not involved with delivering the intervention, who had received training. The majority of the height and weight measurements were taken by this researcher, but where not available, measures taken by one of the clinical researchers delivering the intervention were used in order to maximise the data set. Inter-person variation for the measurements taken by two researchers was found to be non-significant.
Fat mass index and fat free mass index were measured using the 3-component (3C) model which requires measures of total body water (TBW), body volume (BV) and weight. The 3C model was used since it produces similar body composition values in obese children to 4C model, which further incorporates measurement of bone mineral.33 The 3C component model was considered adequate in this study as mineral mass and the protein-mineral ratio were considered unlikely to change over the duration of the intervention. TBW was measured using deuterium oxide dilution and BV by air-displacement plethysmography using BODPOD (Life Measurement Instruments); methods have previously been described in detail.33 Fat mass (FM) and fat free mass (FFM) were derived using established equations34 and index values calculated by dividing each by height squared to take height into account.35
Blood pressure (BP) was measured using a validated electronic sphygmomanometer; three measures were taken and mean diastolic and systolic readings calculated. These were converted into SD scores using UK paediatric reference norms.36 Pubertal status was measured by self-assessment using pictures of the Tanner stages of pubertal development.37
Psychosocial outcomes were measured using questionnaires completed by parents and children. This included measures of self esteem (the Harter scale38), mood (the Children’s Depression Inventory39), parent-reported child difficulties (the Strengths and Difficulties questionnaire, SDQ40), and quality of life (the child- and parent-reported Pediatric Quality of Life Inventory, PedsQL41). Children’s attitudes towards eating and weight were measured using the Children’s Eating Attitudes Test (CHEAT).42-43 Raw scores from the CDI were converted into t-scores for analysis.39 All measures have been validated in children aged 8-12 years.
All outcome measures were taken at baseline and at the end of the 6 month intervention or waiting list period. Additional 12 month anthropometric outcomes were collected for children in the treatment group who completed the programme. Control data are not available past 6 months because waiting list children were subsequently offered treatment. Demographic data collected included child ethnicity and the highest level of parental education which was used as an index of socio-economic status (SES).44
Sample size
A power calculation assuming no clustering was carried out. This was based on the treatment effect of −8.4 (7.1)% of ideal-BMI seen in the pilot groups and an assumed change in the control group of −2.0% with a drop out rate of 30% after recruitment.22 This resulted in the study requiring 48 subjects to be recruited (and a final study sample size of 34; 17 per group) to achieve at least 90% power, α=0.05, using a two-tailed test.
Statistical analysis
Data were analysed using SPSS version 15. Differences in baseline variables between the treatment and control conditions were tested using independent t-tests or Mann-Whitney tests (continuous variables) or chi-squared tests (categorical variables). All 6 month outcomes were analysed on an ITT basis using baseline values carried forward if outcome data were missing.45 The clustering effect of group sessions was found to be extremely small with an intra-class correlation of 0.00017, which meant that it was not necessary to adjust for clustering effects in the analysis.
Outcomes were post-treatment (6 month) data; these were tested for normality using Kolmogorov-Smirnov tests and transformations done as appropriate. Multivariate analysis of covariance (MANCOVA) tests, with age and baseline values as covariates and randomisation group as the fixed factor, were used to test group differences for parametric data. Box’s test was non-significant for all, indicating homogeneity of variance-covariance matrices. Four MANCOVA tests were run: anthropometric outcomes (BMI, weight, height, FM index, and FFM index); anthropometric SDS outcomes (BMI SDS, weight SDS, height SDS, and waist SDS); cardiovascular (CV) outcomes (systolic and diastolic BP SDS); psychosocial outcomes (SDQ; parent and child reported PedsQL; CDI; Harter; CHEAT dieting scale). The total CHEAT score and %BMI were excluded from these analyses to ensure independence of outcomes. Sub-analyses (univariate analysis of covariance) were conducted only where the overall MANCOVA test was significant. The numbers included in the psychosocial outcomes MANCOVA were reduced by missing SDQ data, however analyses excluding the SDQ scores did not change the results (which are not presented). Where data were not normally distributed, Mann Whitney tests were used. Analyses on anthropometric data were also done including pubertal stage as a covariate; this did not change the results and tests adjusting for age and baseline values only are presented.
Paired t-tests or Wilcoxon signed-rank tests were used to examine within group changes over the intervention. No significant demographic differences were found between those with and without complete baseline anthropometric data (11 children had incomplete baseline weight and height data, 3 did not complete baseline questionnaires). One child in the control group was identified as an outlier for BMI SDS, %BMI and BMI change (−0.6, −28.8 and −4.2 respectively; all approximately 4 SD’s of the mean change), so analyses on anthropometric data were additionally run excluding data from this child. Change at 12 months (6 months post-treatment) for children in the treatment group completing treatment was analysed using one-way correlated analysis of variance; only those attending both 6 and 12 month follow-ups were included. Post hoc tests were done using Bonferroni correction; analyses were not adjusted for age.
Results
Participant flow through the trial
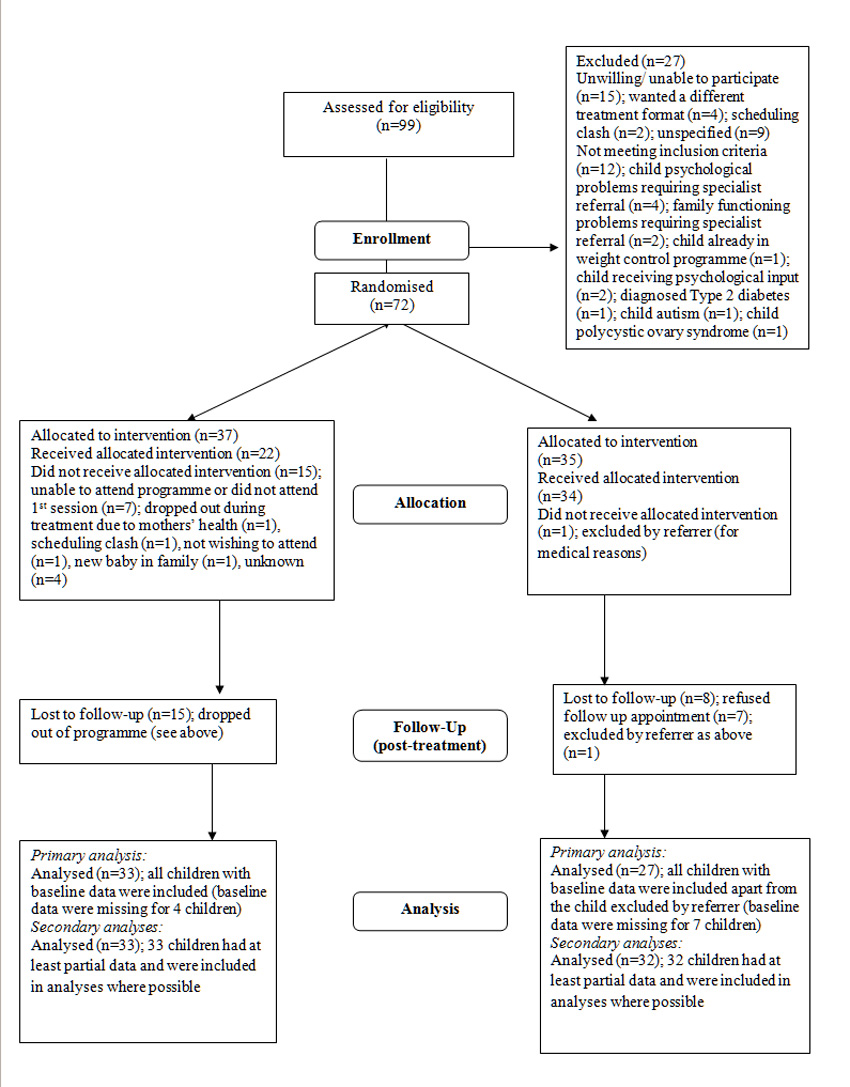
Children were recruited in five waves between January 2004 and June 2007; referrers included GPs, practice nurses, dietitians, and paediatricians. Figure 1 shows the number of children throughout the trial. 22 of the children randomised to the treatment group completed the 6 month intervention (59% of those randomised and 73% of those starting treatment). The median number of sessions attended was 9.0 (IQR 10.50), and 18 (48.6%) children attended 10 or more sessions. 24 children in the control group entered treatment at the end of the waiting list period with 16 completing.
Figure 1.
Participant flow through trial
Baseline characteristics of the sample
Table 1 shows the baseline characteristics of the whole group and by treatment condition. Almost half (43%) the children were non-white and parents had varying levels of education (76% being educated below college level). More girls than boys took part in both groups (approximately 70% vs. 30%). Mean BMI SDS was >3 in both groups, which has been said to indicate extreme obesity.46 There were no significant differences between groups for baseline demographic, psychosocial, body composition, blood pressure, or anthropometric variables apart from age and height, where the treatment group were significantly older and taller than the control group. Children’s quality of life (child and parent-reported PedsQL) was below average,47 but equivalent to obese youth participating in obesity treatment.48 Scores on the global, athletic and physical appearance scales of the Harter were below those reported in Scottish youth.49 Other psychosocial variables were within normal ranges.39-40,43
Table 1. Baseline characteristics of participants.
Figures are means (SD) unless otherwise stated
| Characteristic (number with data if missing data) |
Whole sample (n=72) |
Treatment group (n=37) |
Control group (n=35) |
Test statistic |
P value |
|---|---|---|---|---|---|
| Age (years) | 10.3 (1.6) | 10.8 (1.6) | 9.8 (1.4) | t= 2.8 | 0.006** |
|
| |||||
| Gender % (n) | |||||
| Girls | 69.4 (50) | 70.3 (26) | 68.6 (24) | χ2= 0.02 | 0.9 |
| Boys | 30.6 (22) | 29.7 (11) | 31.4 (11) | ||
|
| |||||
| Parent BMI (n=59) | 30.8 (8.9) | 31.9 (10.5) | 29.3 (6.1) | t= 1.2 | 0.2 |
|
| |||||
| Ethnicity % (n) | |||||
| White | 56.9 (41) | 67.6 (25) | 45.7 (16) | χ2= 5.9 | 0.1 |
| Black | 19.4 (14) | 18.9 (7) | 20.0 (7) | ||
| Asian | 13.9 (10) | 10.8 (4) | 17.1 (6) | ||
| Mixed/ other | 9.7 (7) | 2.7 (1) | 17.1 (6) | ||
|
| |||||
| Parent education % (n) (n=59) | |||||
| Compulsory school education or below | 45.8 (27) | 55.2 (16) | 36.7 (11) | χ2= 3.5 | 0.2 |
| Vocational/ A-level | 30.5 (18) | 31.0 (9) | 30.0 (9) | ||
| Degree or higher | 23.7 (14) | 13.8 (4) | 33.3 (10) | ||
|
| |||||
| Weight (kg) (n=61) | 68.4 (18.3) | 70.8 (17.8) | 65.5 (18.8) | t= 1.1 | 0.3 |
|
| |||||
| Weight SDS (n=61) | 3.1 (0.8) | 3.0 (0.8) | 3.1 (0.8) | t= −0.4 | 0.7 |
|
| |||||
| Height (cm) (n=61) | 148.5 (10.0) | 151.3 (9.3) | 145.1 (9.8) | t= 2.5 | 0.02* |
|
| |||||
| Height SDS (n=61) | 1.4 (1.1) | 1.4 (1.3) | 1.3 (1.0) | t= 0.4 | 0.7 |
|
| |||||
| BMI (n=61) | 30.6 (5.3) | 30.6 (5.1) | 30.6 (5.7) | t= 0.009 | 0.9 |
|
| |||||
| BMI SDS (n=61) | 3.2 (0.6) | 3.1 (0.6) | 3.3 (0.6) | t= −1.0 | 0.3 |
|
| |||||
| % BMI (n=61) | 180.4 (30.6) | 178.3 (29.8) | 182.9 (31.9) | t=−0.6 | 0.6 |
|
| |||||
| Waist (cm) (n=59) | 90.0 (10.4) | 91.2 (9.8) | 88.7 (11.0) | t= 0.9 | 0.3 |
|
| |||||
| Waist SDS (n=59) | 3.4 (0.5) | 3.4 (0.5) | 3.4 (0.6) | t= −0.5 | 0.6 |
|
| |||||
| FM index (kg/m2) (n=60) | 13.4 (3.6) | 13.4 (3.4) | 13.4 (3.8) | t=0.08 | 0.9 |
|
| |||||
| FFM index (kg/m2) (n=60) | 16.9 (2.3) | 16.7 (2.1) | 17.2 (2.5) | t=−0.8 | 0.4 |
|
| |||||
| BP (n=57) | |||||
| Systolic BP SDS | 0.4 (0.9) | 0.3 (0.8) | 0.5 (0.9) | t=−1.0 | 0.3 |
| Diastolic BP SDS | 0.9 (0.8) | 0.8 (0.7) | 1.0 (0.9) | t=−0.6 | 0.6 |
|
| |||||
| Tanner stage % (n) (n=62) |
|||||
| 1 | 45.2 (28) | 41.9 (13) | 48.4 (15) | χ2= 8.1 | 0.1 |
| 2 | 35.5 (22) | 29.0 (9) | 41.9 (13) | ||
| 3 | 8.1 (5) | 16.1 (5) | 0 (0) | ||
| 4 | 8.1 (5) | 6.5 (2) | 9.7 (3) | ||
| 5 | 3.2 (2) | 6.5 (2) | 0 (0) | ||
|
| |||||
| SDQ total score37 (n=41) | 13.2 (6.7) | 12.4 (6.0) | 13.8 (7.4) | t=−0.7 | 0.5 |
|
| |||||
| PedsQL total score38 | |||||
| Child reported (n=61) | 69.4 (16.6) | 70.7 (15.8) | 68.1 (17.6) | t= 0.6 | 0.5 |
| Parent reported (n=59) | 63.3 (15.7) | 64.2 (14.6) | 62.5 (17.0) | t= 0.4 | 0.7 |
|
| |||||
| CDI t-score36 (n=58) | 51.0 (8.9) | 50.8 (7.8) | 51.2 (10.1) | t=−0.1 | 0.9 |
|
| |||||
| Harter global score35 (n=56) | 2.7 (0.7) | 2.7 (0.6) | 2.7 (0.7) | t=−0.3 | 0.8 |
|
| |||||
| CHEAT39-40 (n=62) | |||||
| Total score | 14.0 (9.7) | 13.8 (8.1) | 14.1 (11.2) | t=−0.1 | 0.9 |
| Diet scale | 10.4 (6.9) | 10.5 (6.4) | 10.3 (7.5) | t=0.2 | 0.9 |
| Bulimia/ food preoccupation scale (mdn, IQR) |
0.0 (2.7) | 0.0 (3.0) | 0.0 (2.8) | U=457.5 | 0.9 |
| Oral control scale (mdn, IQR) | 1.0 (3.0) | 1.0 (3.0) | 1.0 (3.0) | U=480.0 | 0.8 |
p<0.05,
p<0.01
BMI=body mass index; FM=fat mass; FFM=fat free mass; BP=blood pressure; SDS=standard deviation score; SDQ=Strengths and Difficulties questionnaire; PedsQL=Pediatric Quality of Life Inventory; CDI=Children’s Depression Inventory; CHEAT=Children’s Eating Attitudes Test; mdn= median; IQR= interquartile range
Between group differences
These are shown in Table 2. There were no significant between group differences for anthropometric SDS, CV or psychosocial outcomes, therefore no further analyses were done for these data. There was a significant effect of treatment group on anthropometric outcomes (p<0.01), but follow up ANOVAs indicated significant group differences for weight and height only, and not for BMI. Non-parametric tests showed no significant differences between groups for the bulimia/ food preoccupation or oral control CHEAT sub-scales. Analyses excluding the outlier in the control group produced a significant effect of treatment on anthropometric outcomes and follow up analyses showed significant differences for height, weight and BMI (shown in Table 2). The treatment effect for the anthropometric SDS outcomes was not significant; Wilks’s Δ= 0.84, F(4,47)=2.24, p=0.08 and further analyses were not done.
Table 2.
Between group changes over the 6 month intervention period
| Outcome variable | Adjusted post intervention mean (sd) | Test statistic F (df) |
P value | |
|---|---|---|---|---|
| Treatment group (n=33) |
Control group (n=30) |
|||
| Anthropometric outcomes |
Wilks’s Δ=0.68
3.53 (6, 44) |
0.006 ** | ||
| BMI | 30.15 (1.17) | 30.61 (1.18) | 1.95 (1, 49) | 0.17 |
| Weight (kg)+ | 66.37 (3.03) | 68.87 (3.06) | 10.46 (1, 49) | 0.002** |
| Height (cm) | 149.92 (1.99) | 151.37 (2.01) | 6.75 (1, 49) | 0.01* |
| Waist | 89.58 (3.28) | 90.48 (3.31) | 0.97 (1, 49) | 0.33 |
| FM index | 13.14 (1.12) | 13.18 (1.13) | 0.02 (1, 49) | 0.90 |
| FFM index | 17.08 (0.77) | 17.27 (0.78) | 0.77 (1, 49) | 0.38 |
|
Anthropometric outcomes
(excl outlier) |
Wilks’s Δ=0.65
3.84 (6, 43) |
0.004 ** | ||
| BMI | 30.11 (0.97) | 30.74 (0.98) | 5.14 (1,48) | 0.03* |
| Weight (kg) + | 66.37 (2.70) | 69.34 (2.73) | 17.51 (1,48) | <0.001** |
| Height (cm) | 150.01 (2.00) | 151.45 (2.02) | 6.41 (1,48) | 0.02* |
| Waist | 89.47 (3.24) | 90.54 (3.28) | 1.35 (1,48) | 0.25 |
| FM index | 13.11 (1.00) | 13.27 (1.01) | 0.35 (1,48) | 0.56 |
| FFM index | 17.09 (0.76) | 17.32 (0.76) | 1.20 (1,48) | 0.28 |
| Anthropometric outcomes (SDS) |
Wilks’s Δ=0.86
1.96 (4, 48) |
0.12 | ||
| BMI SDS+ | 3.11 (0.17) | 3.09 (0.17) | - | - |
| Weight SDS | 2.94 (0.15) | 3.03 (0.16) | - | - |
| Height SDS | 1.23 (0.26) | 1.37 (0.26) | - | - |
| Waist SDS | 3.30 (0.19) | 3.30 (0.19) | - | - |
| Cardiovascular outcomes |
Wilks’s Δ=1.00
0.08 (2,51) |
0.06 | ||
| Systolic BP SDS | 0.16 (0.63) | 0.15 (0.63) | ||
| Diastolic BP SDS | 0.84 (0.82) | 0.77 (0.83) | ||
| Psychosocial outcomes |
Wilks’s Δ=0.82
0.78 (6, 21) |
0.60 | ||
| SDQ total score37;b | 13.30 (3.05) | 12.34 (3.09) | - | - |
| PedsQL total score (parent reported)38;a | 67.60 (10.21) | 68.27 (10.33) | - | - |
| PedsQL total score (child reported)38;a | 70.08 (11.98) | 74.35 (12.12) | - | - |
| CDI t-score36;b | 49.24 (6.91) | 48.13 (6.97) | - | - |
| Harter global score35;a | 2.80 (0.51) | 2.85 (0.51) | - | - |
| CHEAT dieting scale39-40;b | 8.28 (5.16) | 7.60 (5.20) | - | - |
| NP data | Post intervention median (IQR) | U | P value | |
| CHEAT bulimia/ food preoccupation scale39-40;b |
0.00 (2.00) | 0.00 (3.00) | 517.00 | 0.14 |
| CHEAT oral control scale39-40;b | 1.00 (3.00) | 1.50 (3.25) | 457.50 | 0.70 |
p<0.05,
p<0.01;
ITT analyses conducted using baseline values carried forward; post intervention mean values adjusted for age (in months) and baseline levels; main analyses were MANCOVA tests, sub analyses only carried out where significant differences seen at MANCOVA level. Numbers if data missing, for treatment and control group respectively, are: anthropometric, n=31 and n=27; CV, n=29 and n=28; psychosocial, n=20 and n=15;
Reported means are anti-logs of the mean of the logged data, standard deviations of the original data are given;
Increased score indicates an improvement;
Decreased score indicates an improvement;
Abbreviations are BMI=body mass index; FM=fat mass; FFM=fat free mass; BP=blood pressure; SDS=standard deviation score; SDQ=Strengths and Difficulties questionnaire; PedsQL=Pediatric Quality of Life Inventory; CDI=Children’s Depression Inventory; CHEAT=Children’s Eating Attitudes Test; NP=non parametric; IQR= interquartile range
Within group changes
These are shown in Table 3. Both groups experienced significant reductions in BMI SDS (p<0.01), but not BMI. There were significant increases in weight and height, and reductions in weight SDS, % BMI and waist SDS for both treatment conditions (p<0.05), and a significant reduction in height SDS for the treatment group. There were no significant changes in waist or body composition for either group. FFM index increased marginally in both groups (within group analyses; p=0.09), indicating that the process of growth and maturation changed children’s physique during the intervention period for both groups. Systolic BP SDS reduced significantly in the treatment group (p<0.05). Parent-reported quality of life improved in the treatment group, and there were improvements on the total CHEAT score and the CHEAT dieting and bulimia/ food preoccupation subscales (p<0.05). The global Harter score improved marginally for the treatment group (p=0.05). There were no significant changes for any measure in the control group.
Table 3.
Within group changes over the 6 month intervention period
| Outcome variable | Within group adjusted mean change (SD) |
|
|---|---|---|
| Treatment group (n= 33) | Control group (n=30) | |
| BMI | −0.36 (1.06) | −0.03 (1.07) |
|
| ||
| BMI SDS | −0.11 (0.16)** | −0.10 (0.16)** |
|
| ||
| BMI (excluding outlier) | −0.35 (0.90) | +0.11 (0.91) |
|
| ||
| BMI SDS (excluding outlier) | −0.11 (0.14)** | −0.09 (0.14)** |
|
| ||
| % BMI | −3.74 (6.44)** | −2.88 (6.47)* |
|
| ||
| Weight (kg) | +0.79 (2.84)* | +2.78 (2.85)** |
|
| ||
| Weight SDS | −0.09 (0.15)** | −0.04 (0.15)* |
|
| ||
| Height (cm) | +1.67 (1.83)** | +3.11 (1.84)** |
|
| ||
| Height SDS | −0.005 (0.14)** | +0.07 (0.14) |
|
| ||
| Waist (cm) | −0.51 (3.23) | +0.18 (3.24) |
|
| ||
| Waist SDS | −0.09 (0.19)** | −0.10 (0.20)** |
|
| ||
| FM index (kg/m2) | −0.15 (1.07) | −0.21 (1.07) |
|
| ||
| FFM index (kg/m2) | 0.15 (0.80) | 0.33 (0.80) |
|
| ||
| Systolic BP SDS | −0.24 (0.71)* | −0.30 (0.71) |
|
| ||
| Diastolic BP SDS | −0.03 (0.86) | −0.14 (0.86) |
|
| ||
| SDQ total score37b | −1.07 (4.22) | −0.42 (4.23) |
|
| ||
| PedsQL total score38a | ||
| Parent reported | +3.81 (9.08)* | +3.02 (9.10) |
| Child reported | +0.84 (11.79) | +4.01 (11.80) |
|
| ||
| CDI t-score36b | −1.80 (6.31) | −1.45 (6.33) |
|
| ||
| Harter global score35a | +0.20 (0.64)+ | +0.14 (0.64) |
|
| ||
| CHEAT39-40b | ||
| Total score | −2.30 (6.41)* | +0.17 (6.42) |
| Dieting scale | −1.70 (4.86)* | −0.81 (4.86) |
| Bulimia/ food | −0.76 (1.76)* | +0.18 (1.76) |
| preoccupation scale | ||
|
| ||
| Oral control scale | +0.20 (2.20) | +0.76 (2.20) |
p<0.05,
p<0.01,
p=0.05 (using paired t-tests or Wilcoxon signed-rank tests);
ITT analyses conducted using baseline values carried forward; change values adjusted for age (in months); outlier was a child in the control group; Numbers if data missing, for treatment and control group respectively, are: weight, height and BMI, n=27 for the control group; waist, n=31 and n=27; body composition, n=31and n=28; BP, n=29 and n=28; SDQ, n=21 and n=22; Harter, n=30 and n=26; CHEAT/ PedsQL/ CDI, n=31 and n=26;
Increased score indicates an improvement;
Decreased score indicates an improvement: Abbreviations are: BMI=body mass index; FM=fat mass; FFM=fat free mass; BP=blood pressure; SDS=standard deviation score; SDQ=Strengths and Difficulties questionnaire; PedsQL=Pediatric Quality of Life Inventory; CDI=Children’s Depression Inventory; CHEAT=Children’s Eating Attitudes Test
Children’s BMI SDS change varied considerably over the intervention period. In the treatment group BMI SDS changes ranged from −0.53 to +0.14, and in the control group from −0.58 to +0.10.
Longer term outcomes for the treatment group
6 month post-intervention follow-up data for the treatment group who completed the intervention are shown in Table 4. Change in BMI SDS over the treatment period was maintained at 6 month follow-up. Change in BMI over treatment was significant here, where it had not been for the ITT analysis, but was not maintained at follow up. However, there was no overall change in BMI or BMI SDS between baseline and 12 months. The lack of effect in level of overweight at 12 months appears due to the significant amount of weight gained between 6 and 12 months. The waist SDS reduction over treatment was more or less maintained over follow-up.
Table 4.
Completers’ analysis for the treatment group over the intervention and follow up period
| Variable | Mean (SD) |
Post hoc tests (mean difference) |
||||||
|---|---|---|---|---|---|---|---|---|
| T1 (0 months) |
T2 (6 months) |
T3 (12 months) |
F | P value | T1-T2 | T2-T3 | T1-T3 | |
| BMI (n=19) |
30.86 (5.55) | 30.30 (5.67) | 31.37 (5.62) | 5.37 | 0.02* | −0.56* | +1.07* | +0.51 |
|
| ||||||||
| BMI SDS (n=19) |
3.14 (0.72) | 2.98 (0.75) | 3.03 (0.78) | 6.18 | 0.005** | −0.16** | +0.06 | −0.11 |
|
| ||||||||
| Weight (kg) (n=19) |
71.50 (19.32) | 72.54 (19.62) | 77.15 (19.87) | 21.63 | 0.00** | +1.04 | +4.61** | +5.65** |
|
| ||||||||
| Weight SDS (n=19) |
3.10 (0.99) | 2.98 (1.01) | 3.01 (1.02) | 2.92 | 0.09 | −0.12** | +0.03 | −0.10 |
|
| ||||||||
| Waist (cm) (n=17) |
92.00 (10.42) | 91.32 (10.17) | 92.88 (10.93) | 1.38 | 0.27 | −0.68 | +1.55 | +0.88 |
|
| ||||||||
| Waist SDS (n=17) |
3.53 (0.45) | 3.42 (0.48) | 3.41 (0.49) | 3.36 | 0.047* | −0.11+ | −0.01 | −0.12+ |
p<0.05,
p<0.01,
p<0.08 BMI=body mass index; SDS=standard deviation score; Post hoc tests done using Bonferroni correction
Adverse outcomes
We were not aware of any specific adverse health consequences for participating children, although one child in the control group reduced their BMI by 28.8 and BMI SDS by 4.2.
Discussion
In this RCT of comprehensive FBBT to treat childhood obesity, the main effect (between group differences in post-treatment BMI and BMI SDS) was not significant. There were also no significant differences between treatment and control groups for any of the secondary anthropometric or body composition outcomes, apart from weight and height which were greater in controls post-treatment. There were significant within group increases in both groups for weight and height, and reductions for BMI SDS, % BMI, weight SDS, and waist SDS, although not for BMI, waist, FM or FFM (although there was a trend for increases in the latter). Excluding an outlier from the control group resulted in a significant between group difference for BMI. BP SDS change was similar in the two groups, although systolic BP SDS change was only significant for the treatment group. Another UK childhood obesity intervention produced significant anthropometric and CVD changes50 with no body composition changes (Wells, unpublished data), indicating the potential to modify CVD risk factors in the absence of body composition change. However, others have shown that a BMI SDS of ≥0.25 is needed for a clinically significant impact on CVD risk factors.51-52
The between group differences for the psychosocial outcomes were not significant, although within group tests suggested that overall children’s well-being improved on participating in FBBT, with few changes for the control group. For children completing treatment (n=22), mean BMI SDS change was −0.16 (SD 0.17), which is very similar to the pilot study.22 There was limited evidence of a longer-term benefit; BMI SDS and waist SDS reductions were partially maintained at 12 month follow up, although overall, changes between baseline and follow up were not significant. BMI rebounded post-treatment, with significant increases from 6 to 12 months.
In order to address previous criticisms of research quality in this area13, 25 we used a rigorous design with ITT analyses, and the study was powered to detect changes in the primary outcome measures of a magnitude comparable to those observed in our pilot data.22 FBBT has sound theoretical underpinning, is well described in the literature, and in the present study, clinicians adhered to a treatment protocol. We used a range of validated psychosocial measures and anthropometric data were objectively measured. Few data regarding body composition in obese children have been published, especially across treatment. Our sample was socially and ethnically diverse addressing a criticism of FBBT research over lack of generalisability.
There were limitations to the study. Retention in children attending the intervention was modest (59%), although comparable to other studies53. Full baseline data were not available for 11 children, due to non-attendance at assessment appointments, therefore we could not include them in the analyses. Given the nature of the intervention, it was not possible to blind families or researchers/clinicians to group allocation. Because of cost restraints, we were not able to formally evaluate fidelity to the protocol, but the same core group of clinicians carried out all treatments, and the programme had been manualised. Since we used a waiting list control design, we do not have any long-term follow-up data in the control group and are unable to show whether reductions in BMI SDS were sustained without further input. Additionally, ITT analyses were only used for the 6 months outcomes.
These results contrast with the conclusions of the Cochrane review which found a significant pooled treatment effect of −0.06 BMI SDS from behavioural interventions compared to usual care.13 However, there are problems in comparing outcomes between studies; BMI has been proposed as a better measure than BMI SDS or %BMI since it has greater sensitivity in identifying change in very overweight children.29 Additionally, the reference data used to calculate BMI SDS and %BMI vary because of use of country-specific data. Only three RCTs have been published in the UK. The Scottish Childhood Overweight Treatment Trial (SCOTT) produced similar BMI SDS changes (−0.10 in the intervention group) with no significant difference between control and intervention groups.54 The Bristol Care of Childhood Obesity Clinic (COCO) used a novel approach to slow down eating rate and found a reduction in BMI SDS of −0.36 after 12 months in those attending follow up, regardless of attendance over treatment.55 The ‘Mind, Exercise, Nutrition…. Do it’ (MEND) programme reported a higher BMI SDS reduction at 6 months than the current study, but these results are not comparable because ITT analyses were not used.50 The 12 month anthropometric reductions in waist, waist SDS and BMI SDS were all significant for children attending MEND and were considerably larger than here.
Epstein and colleagues have published the outcomes of numerous studies of FBBT with impressive average BMI SDS changes of −1.20 at 6 months and −1.02 at 12 months, although direct comparisons with our data are problematic given the use of different reference data.56 Additionally, the majority of these results come from completers’ data, which is likely to inflate effects compared with an ITT analysis. However, one study reported % overweight changes over treatment using ITT analyses and found changes of −22.7% at 6 months and −10.9% at 12 months, compared with −25.5% and −12.9% using completers’ data.14 It is therefore clear that our outcomes are considerably less good than those achieved by Epstein’s group, although comparable to some of the other UK studies.
The lack of treatment effect seems to be a combination of equivalent change in the control condition and modest change in the treatment group. Children in the control group were a help-seeking population and therefore unlikely to be representative of ‘typical’ obese children in the community, although one review reported that children in waiting list control groups experienced increases in overweight; at odds with our findings.57 Families received considerable input prior to randomisation which may have been sufficient to engender some change. Indeed single session motivational interviewing has produced positive outcomes.58 The treatment group was significantly older than the control group at baseline and also grew less in height. Differences between the groups may therefore be underestimated, although repeating the analyses with pubertal stage as a covariate did not alter the findings.
Given the strong evidence base for FBBT, the modest effect requires explanation. We aimed to be faithful to Epstein’s FBBT and delivered the programme as planned, however there were important differences. Because of considerable resistance from families and health professionals, we changed calorie goals to goals on food types and portion size, removed goals for daily weighing, and did not incentivise participation.14-15,22 These changes could have diluted the programme’s impact although others have used similar dietary goals and seen a larger treatment effect than we did.59 Our sample was ethnically diverse, with 43% being non-white, possibly influencing acceptability and effectiveness. The high prevalence of overweight in the parents and high level of psychosocial difficulties in the children in our sample could also have diminished the outcomes.60-61 The optimal setting for obesity treatments is unclear; Epstein’s studies took place in a university setting whereas ours was incorporated into an NHS service and programme efficacy may be reduced in this less controlled environment.56
There have been suggestions that solely targeting parents could enhance intervention effectiveness, and such interventions have produced BMI SDS reductions in excess of −0.2 BMI SDS in ITT analyses.59,62-63 One of these studies included a no treatment control group; between group differences were non-sigificant.63 It is difficult to disentangle the impact of the intervention target since comparison groups vary between studies; two studies compared parent-targeted ‘parenting’ and ‘lifestyle’ components.59,63 whereas another compared parents alone vs. parents and children attending all sessions together.62 FBBT on the other hand, as delivered here and by Epstein, targeted parents and children but included parent-only sessions. The children in the parent-only interventions also tend to be younger than the current study.
In conclusion, this study did not find significant between-group differences in the change in children’s level of overweight (indexed by BMI or BMI SDS) between treatment and control groups. BMI SDS (although not BMI) changes were significant but similar in both groups. There were also no between-group treatment effects on secondary outcomes. The results of this study raise questions over the generalisability of FBBT and the use of an expensive treatment when other less intense and less expensive options are available, although given the success of the programme in Epstein’s group, it would be useful for other systematic evaluations to further test the generalisability of FBBT. There were large individual differences in weight change, with some children being very successful. One important future area of work would therefore be to identify family characteristics that increase the likelihood of success. It is also possible that FBBT could be more acceptable to those with psychosocial difficulties (albeit with the possibility of poorer outcomes, as discussed above) whose needs may be too complex for non-specialist programmes; again future research could explore this. The level of input received by the control group may have influenced families’ behaviour and diluted the observed impact of the intervention. This raises the possibility that brief interventions based on motivational interviewing could offer an alternative, and less expensive, approach to managing at least some cases of childhood obesity.
Supplementary Material
Acknowledgements
We would like to thank the families who took part, and the clinical and administrative staff at GOSH and research staff at UCL for contributing their time and effort to the study. The trial was funded by Cancer Research UK, Great Ormond Street Hospital and Weight Concern. All authors contributed to the study design and protocol. JW obtained the funding for the study. HC was responsible for the day-to-day running of the study and for recruitment, supervised the running of treatment sessions and clinical staff, analysed the data, interpreted the results and drafted the paper. RMV was responsible for the medical assessment and care of children, analysed data and interpreted results. DN had overall clinical responsibility for families, analysed data and interpreted results. DH carried out the body composition and anthropometric measurements. PC and CE contributed to the supervision of treatment sessions and clinical staff. JCKW provided the protocol and facilities for the body composition measures. All authors reviewed the manuscript. JW is guarantor.
This research was funded by Cancer Research UK, Great Ormond Street Hospital and Weight Concern
Footnotes
Conflicts of interest There are no conflicts of interest for any of the authors.
Supplemental information An outline of the contents of the parent and child sessions is provided in Supplemental Table 1. This is available at…
This trial was registered at http://www.controlled-trials.com/ as ISRCTN 51382628
References
- (1).Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ode KL, Frohnert BI, Nathan BM. Identification and treatment of metabolic complications in pediatric obesity. Rev Endocr Metab Disord. 2009;10:167–88. doi: 10.1007/s11154-009-9115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: A systematic review. Int J Pediatr Obes. 2010;5:282–304. doi: 10.3109/17477160903473697. (2010) [DOI] [PubMed] [Google Scholar]
- (4).Singh AS, Mulder C, Twisk JWR, van Mechelen W, Chinapaw MJM. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- (5).The NHS Information Centre Health Survey for England- 2008 trend tables. 2009 available at www.ic.nhs.uk/pubs/hse08trends.
- (6).Korsten-Reck U, Kromeyer-Hauschild K, Wolfarth B, Dickhuth HH, Berg A. Freiburg Intervention Trial for Obese Children (FITOC): results of a clinical observation study. Int J Obes Relat Metab Disord. 2004;29:356–361. doi: 10.1038/sj.ijo.0802875. [DOI] [PubMed] [Google Scholar]
- (7).Reinehr T, de SG, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr. 2006;84:490–496. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- (8).Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- (9).Jiang JX, Xia XL, Greiner T, Lian GL, Rosenqvist U. A two year family based behaviour treatment for obese children. Arch Dis Child. 2005;90:1235–1238. doi: 10.1136/adc.2005.071753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Barton SB, Walker LL, Lambert G, Gately PJ, Hill AJ. Cognitive change in obese adolescents losing weight. Obes Res. 2004;12:313–319. doi: 10.1038/oby.2004.39. [DOI] [PubMed] [Google Scholar]
- (11).Braet C, Tanghe A, Decaluwe V, Moens E, Rosseel Y. Inpatient Treatment for Children With Obesity: Weight Loss, Psychological Well-being, and Eating Behavior. J Pediatr Psychol. 2004;29:519–529. doi: 10.1093/jpepsy/jsh054. [DOI] [PubMed] [Google Scholar]
- (12).Epstein LH, Paluch RA, Saelens BE, Ernst MM, Wilfley DE. Changes in eating disorder symptoms with pediatric obesity treatment. J Pediatr. 2001;139:58–65. doi: 10.1067/mpd.2001.115022. [DOI] [PubMed] [Google Scholar]
- (13).Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;1 doi: 10.1002/14651858.CD001872.pub2. CD001872. [DOI] [PubMed] [Google Scholar]
- (14).Epstein LH, Paluch RA, Gordy CC, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Arch Pediatr Adolesc Med. 2000;154:220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- (15).Epstein LH, Paluch RA, Gordy CC, Saelens BE, Ernst MM. Problem solving in the treatment of childhood obesity. J Consult Clin Psychol. 2000;68:717–721. [PubMed] [Google Scholar]
- (16).Epstein LH, McCurley J, Wing RR, Valoski A. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol. 1990;58:661–664. doi: 10.1037//0022-006x.58.5.661. [DOI] [PubMed] [Google Scholar]
- (17).Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- (18).Gunnarsdottir T, Njardvik U, Olafsdottir AS, Craighead L, Bjarnason R. Childhood obesity and co-morbid problems: effects of Epstein’s family-based behavioural treatment in an Icelandic sample. J Eval Clin Pract. 2011 doi: 10.1111/j.1365-2753.2010.01603.x. DOI: 10.1111/j.1365-2753.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- (19).Herrera EA, Johnston CA, Steele RG. A comparison of cognitive and behavioral treatments for pediatric obesity. Child Health Care. 2004;33:151–167. [Google Scholar]
- (20).Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioural weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30:318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- (21).Epstein LH. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord. 1996;20(suppl 1):S14–S21. [PubMed] [Google Scholar]
- (22).Edwards C, Nicholls D, Croker H, Van ZS, Viner R, Wardle J. Family-based behavioural treatment of obesity: acceptability and effectiveness in the UK. Eur J Clin Nutr. 2006;60:587–592. doi: 10.1038/sj.ejcn.1602353. [DOI] [PubMed] [Google Scholar]
- (23).Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Food Standards Agency . In: eatwell plate. Crown Copyright, editor. 2007. [Google Scholar]
- (28).O’Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci. 2010;28:573–591. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- (29).Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- (30).Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross-sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–29. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).McCarthy HD, Jarrett KV, Crawley HF. The development of waist circumference percentiles in British children aged 5.0-16.9 y. Eur J Clin Nutr. 2001;55:902–907. doi: 10.1038/sj.ejcn.1601240. [DOI] [PubMed] [Google Scholar]
- (33).Haroun D, Wells JCK, Williams JE, Fuller NJ, Fewtrell MS, Lawson MS. Composition of the fat-free mass in obese and nonobese children: matched case-control analyses. Int J Obes. 2005;29:29–36. doi: 10.1038/sj.ijo.0802834. [DOI] [PubMed] [Google Scholar]
- (34).Wells JCK, Fuller NJ, Fewtrell MS, Elia M, Cole TJ. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- (35).VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–599. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- (36).Jackson LV, Thalange NK, Cole TJ. Blood pressure centiles for Great Britain. Arch Dis Child. 2007;92:298–303. doi: 10.1136/adc.2005.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatr. 1980;66:918–920. [PubMed] [Google Scholar]
- (38).Harter S. The Perceived Competence Scale for Children. Child Dev. 1982;53:87–97. [Google Scholar]
- (39).Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–315. [PubMed] [Google Scholar]
- (40).Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- (41).Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- (42).Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- (43).Maloney MJ, McGuire JB, Daniels SR. Reliability testing of a children’s version of the Eating Attitude Test. J Am Acad Child Adolesc Psychiatry. 1988;27:541–543. doi: 10.1097/00004583-198809000-00004. [DOI] [PubMed] [Google Scholar]
- (44).Gibson EL, Wardle J, Watts CJ. Fruit and vegetable consumption, nutritional knowledge and beliefs in mothers and children. Appetite. 1998;31:205–228. doi: 10.1006/appe.1998.0180. [DOI] [PubMed] [Google Scholar]
- (45).Ware JH. Interpreting incomplete data in studies of diet and weight loss. NEJM. 2003;348:2136–2137. doi: 10.1056/NEJMe030054. [DOI] [PubMed] [Google Scholar]
- (46).Viner R, Nicholls D. Managing obesity in secondary care: a personal practice. Arch Dis Child. 2005;90:385–390. doi: 10.1136/adc.2004.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- (48).Rudolf M, Christie D, McElhone S, Sahota P, Dixey R, Walker J, et al. WATCH IT: a community based programme for obese children and adolescents. Arch Dis Child. 2006;91:736–739. doi: 10.1136/adc.2005.089896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Hoare P, Elton A, Greer A, Kerley S. The modification and standardisation of the Harter self-esteem questionnaire with Scottish school children. Eur Child Adolesc Psychiatry. 1993;2:19–33. doi: 10.1007/BF02098827. [DOI] [PubMed] [Google Scholar]
- (50).Sacher PM, Kolotourou M, Chadwick PM, Cole TJ, Lawson MS, Lucas A, et al. Randomized Controlled Trial of the MEND Program: A Family-based Community Intervention for Childhood Obesity. Obesity. 2010;18(suppl 1):S62–S68. doi: 10.1038/oby.2009.433. [DOI] [PubMed] [Google Scholar]
- (51).Ford AL, Hunt LP, Cooper A, Shield JPH. What reduction of BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95:256–261. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- (52).Reinehr T, Adler W. Changes in the atherogenic risk profile according to degree of weight loss. Arch Dis Child. 2004;89:419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00803.x. doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Hughes AR, Stewart L, Chapple J, McColl JH, Donaldson MDC, Kelnar CJH, et al. Randomized, controlled trial of a best-practice individualized behavioral program for treatment of childhood overweight: Scottish Childhood Overweight Treatment Trial (SCOTT) Pediatr. 2008;121:e539–e546. doi: 10.1542/peds.2007-1786. [DOI] [PubMed] [Google Scholar]
- (55).Ford AL, Bergh C, Sodersten P, Sabin MA, Hollinghurst S, Hunt LP, et al. Treatment of childhood obesity by retraining eating behaviour: randomised controlled trial. BMJ. 2009;340:b5388. doi: 10.1136/bmj.b5388. [DOI] [PubMed] [Google Scholar]
- (56).Epstein LH, Paluch R, Roemmich J, Beecher M. Family-based obesity treatment, then and now: twenty five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wifley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomised controlled trials. Health Psychol. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- (59).Magarey AM, Perry RA, Baur LA, Steinbeck KS, Sawyer M, Hills AP, et al. A parent-led family-focused treatment program for overweight children aged 5 to 9 years: the PEACH RCT. Pediatrics. 2011;127:214–22. doi: 10.1542/peds.2009-1432. [DOI] [PubMed] [Google Scholar]
- (60).Epstein LH, Wing RR, Koeske R, Valoski A. Effect of parent weight on weight loss in obese children. J Consult Clin Psychol. 1986;54:400–401. doi: 10.1037//0022-006x.54.3.400. [DOI] [PubMed] [Google Scholar]
- (61).White MA, Martin PD, Newton RL, Walden HM, York-Crowe EE, Gordon ST, et al. Mediators of weight loss in a family-based intervention presented over the internet. Obes. 2004;12:1050–1059. doi: 10.1038/oby.2004.132. [DOI] [PubMed] [Google Scholar]
- (62).Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95:1008–15. doi: 10.1079/bjn20061757. [DOI] [PubMed] [Google Scholar]
- (63).Golley RK, Magarey AM, Baur LA, Steinbeck KS, Daniels LA. Twelve-month effectiveness of a parent-led, family-focused weight-management program for prepubertal children: a randomized, controlled trial. Pediatrics. 2007;119:517–25. doi: 10.1542/peds.2006-1746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.