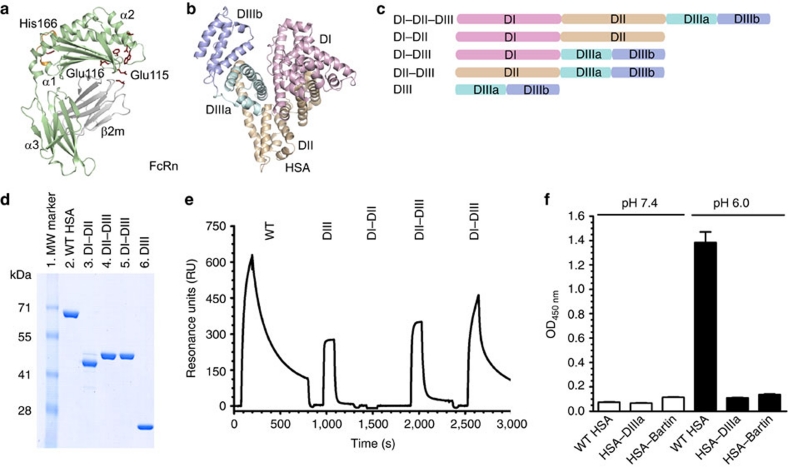
Figure 1. Domain architecture of HSA and hFcRn binding properties of HSA hybrid molecules.
(a) Overall structure of hFcRn showing the location of the pH-dependent flexible loop (orange) and His-166 relative to the IgG binding site (red residues in ball-and-stick)23. (b) The crystal structure of full-length HSA shows three α-helical domains; DI (pink), DII (orange) and DIII (cyan/blue)20. The DIII is split into subdomains DIIIa (cyan) and DIIIb (blue). (c) Domain organization of constructed hybrid HSA molecules (DI–DII, DI–DIII, DII-DIII, DIII). (d) SDS–PAGE gel migration of the HSA domain variants. (e) Representative SPR sensorgrams of equal amounts of WT HSA and domain combinations injected over immobilized hFcRn at pH 6.0. (f) ELISA showing pH-dependent binding of 5 μg ml−1 of WT HSA, HSA–DIIIa and HSA–Bartin to hFcRn at pH 7.4 and pH 6.0. n=4. All data are presented as mean±s.d.