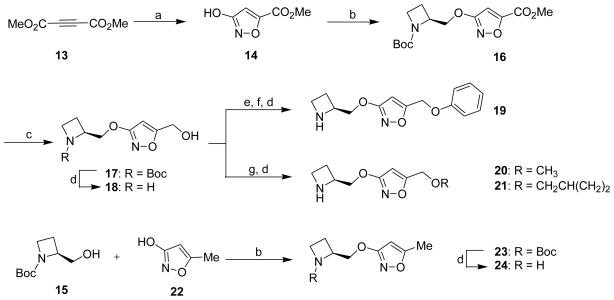
Scheme 1a.
a Reagents and conditions: (a) N-hydroxyurea, 1,5-diazabicyclo[5.4.0]undec-5-ene, MeOH, 0 °C, then HCl; (b) 1-(tert-butoxycarbonyl)-2(S)-azetidinylmethanol (15), diisopropyl azodicarboxylate, PPh3, THF, 0 °C to rt; (c) LiBH4, THF, 0 °C to rt; (d) TFA, CH2Cl2; (e) I2, PPh3, imidazole, CH2Cl2, 0 °C to rt; (f) phenol, K2CO3, DMF, rt; (g) NaH, DMF, RBr, 0 °C to rt.