Table 2.
Binding affinities of 5-alkoxyisoxazole ligands at seven rat nAChR subtypes
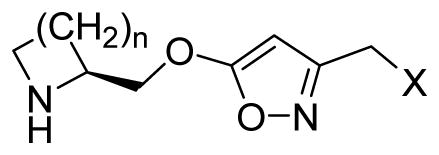 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | n | X |
Ki (nM)a |
||||||||
| α2β2 | α2β4 | α3β2 | α3β4 | α4β2 | α4β2*b | α4β4 | α7 | α7*b | |||
| 31 | 1 | OH | 197 | >104 | 521 | >104 | 137 | 637 | 4900 | NDd | ND |
| 32 | 1 | OC6H5 | 47.9 | 58.0 | 362 | 186 | 23.8 | 172 | 27.5 | ND | ND |
| 33 | 2 | OC6H5 | 176 | 320 | 2040 | 809 | 160 | 2120 | 55.1 | ND | ND |
| 34 | 1 | NHC6H5 | 150 | 144 | 462 | 771 | 75.9 | 386 | 33.2 | ND | ND |
| 35 | 1 | NHC6H4F-p | 201 | 30.3 | ND | 171 | 49.0 | 417 | 9.9 | ND | ND |
| 36 | 1 | OC(O)NHC6H5 | 42.3 | 162 | 123 | 1760 | 19.7 | 157 | 60.0 | ND | ND |
| 37 | 1 | OC(O)NHC2H5 | 157 | 6570 | 315 | NAe | 31.2 | 207 | 1240 | ND | ND |
| 38 | 1 | OC(O)NH-c-C5H11 | 126 | 9970 | 370 | NA | 13.1 | 149 | 3480 | ND | ND |
| 39 | 1 | F | 11.8 | 472 | 17.3 | 1270 | 7.3 | 11.9 | 163 | ND | ND |
| 43 | 1 | H | 4.3 | 311 | 8.7 | 692 | 4.6 | 12.0 | 86.0 | 2890 | 6790 |
| 44 | 2 | H | 616 | 5810 | 1030 | 8780 | 129 | 1100 | 4140 | ND | ND |
| 3c | - | - | 5.5 | 70.0 | 29.0 | 260 | 4.9 | 9.8 | 23.0 | ND | ND |
| 1f | - | - | - | - | - | 86 | 0.4 | - | 110 | 125 | - |
See Experimental Section.
α4β2* or α7*, endogenous receptors prepared from rat forebrain. Besides α4, β2 or α7, other unidentified subunits may also be present. Details are provided in the Experimental Section.
The Ki values for compound 3 are taken from the PDSP Assay Protocol Book.
ND: not detected.
not active, defined as < 50% binding in the primary assay at 10 μM.
The Ki values for compound 1 are from reference 51.
