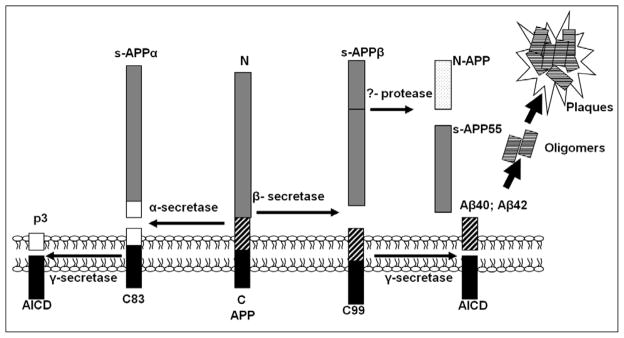
Figure 1. Summary of the proteolytic processing of APP.
The early-onset familiar AD gene APP encodes amyloid β-protein precursor, which generates Aβ through the serial proteolytic cleavage by β- and γ-secretase. β-secretase cleavage produces the secreted, ~90kDa protein, sAPPβ, and the β-carboxy-terminal fragment, β-CTF (or C99). sAPPβ is the substrate of an unidentified secretase, which produces N-APP (containing the N-terminal 286 amino acids of APP; ~35kDa) and s-APP55 (~55kDa). C99 can be cleaved by γ-secretase and gives rise to Aβ and AICD (APP intracellular domain). In contrast to this amyloidogenic process by β- and γ-secretase, APP undergoes an alternative cleavage pathway which precludes Aβ generation. This pathway is initiated by α-secretase and produces sAPPα and the carboxy-terminal fragment, α-CTF (or C83). C83 can be further cleaved by γ-secretase to produce P3 and AICD.