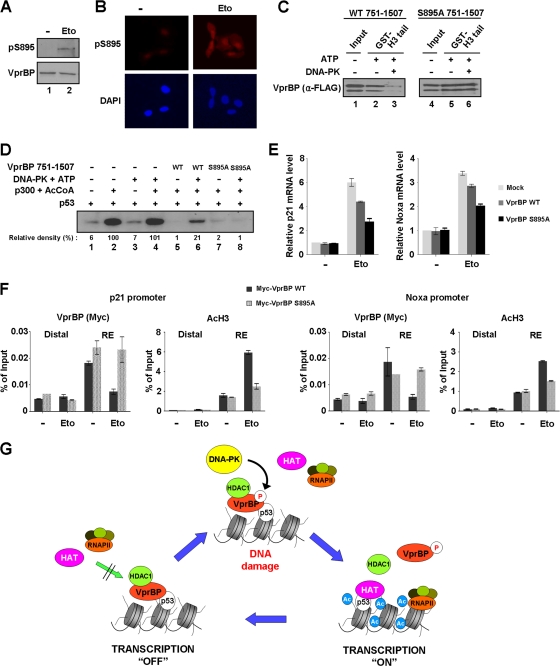
Fig 9.
Alleviation of VprBP-induced repression by phosphorylation. (A) Western blotting of S895 phosphorylation after DNA damage. Cell extracts were prepared 8 h after etoposide treatment of U2OS cells and analyzed by Western blot analysis using VprBP and phospho-S895 VprBP antibodies. (B) Immunostaining of S895 phosphorylation after DNA damage. U2OS cells were treated with etoposide as for panel A and stained with phospho-S895 VprBP antibody. Increased staining for S895 phosphorylation is evident after etoposide-induced DNA damage. (C) Impaired interaction of VprBP with H3 tail upon phosphorylation. In vitro binding experiments using immobilized H3 tails were performed with phosphorylated or unphosphorylated wild type (WT) (lanes 1 to 3) and S895-mutated (lanes 4 to 6) VprBP. VprBP binding to H3 tails was determined by Western blotting using anti-Flag antibody. (D) Stimulatory effect of VprBP phosphorylation on transcription. Transcription reactions were identical to those in Fig. 3C, but wild-type and mutant VprBP 751–1507 fragments were premodified by DNA-PK. (E) Differential effects of wild-type and S895-mutated VprBP on p21 and Noxa transcription. U2OS cells were transfected with Myc-tagged versions of wild-type and S895-mutated VprBP for 48 h and treated with etoposide for additional 12 h. p21 and Noxa mRNA levels were analyzed by using qRT-PCR. (F) Phosphorylation-dependent dissociation of VprBP from the promoter. U2OS cells were transfected with wild-type and S895-mutated VprBP as for panel E. ChIP assays after etoposide treatment were essentially as described for Fig. 5B. (G) Model for VprBP inhibition of p53-mediated transcription. Under unstressed conditions, VprBP is brought to p53-responsive promoters by p53 and acts as a molecular rheostat to block transcription initiation by binding to unacetylated H3 tails. For the most efficient repression, VprBP cooperates with HDAC1 to remove and block H3 acetylation. Upon DNA damage, the majority of VprBP is phosphorylated by DNA-PK and dissociated from the promoter nucleosomes. HAT then acetylates H3 tails and establishes an active promoter environment for p53 to achieve the most efficient transcription of proapoptotic genes. In this way, cells would achieve more accurate and efficient regulation of the p53 transcriptional network. See Discussion for further details.