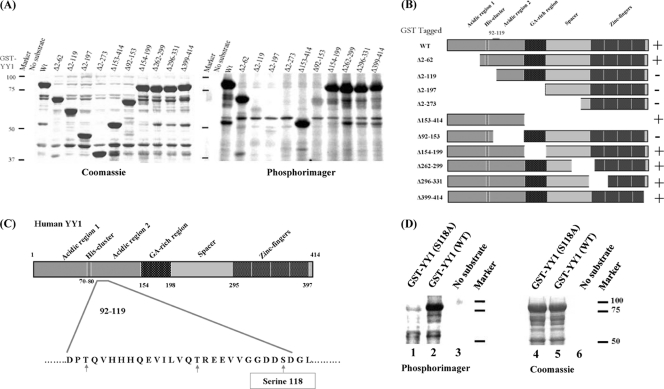
Fig 2.
CK2α phosphorylation of YY1 in its transactivation domain in vitro. (A) GST-tagged YY1 deletion mutants used in the radioactive in vitro kinase reactions are indicated above the lanes. The no-substrate kinase reaction served as a negative control to eliminate the possibility of CK2α autophosphorylation. Kinase reaction mixtures were separated on a 10% SDS-PAGE gel, and the gel was stained with Coomassie blue (left) to visualize the protein bands, dried, and incubated overnight with a phosphorimager screen (right) at room temperature. The screen was then scanned on a Typhoon 9410 imager (Amersham Biosciences). (B) Diagram of GST-YY1 (WT) and GST-YY1 deletion mutants (6) used in the kinase reactions shown in panel A. The region between amino acids 92 and 119, shown on the full-length YY1, is the region identified as the site of CK2α phosphorylation. + and − indicate the presence or absence of phosphorylation. (C) Diagram of the different domains of the YY1 protein. Amino acid residues 92 to 119 are shown; serine and threonine residues within this amino acid sequence are indicated by arrows. Serine 118, marked in a rectangle, is the best candidate residue for CK2α phosphorylation. (D) Radioactive in vitro kinase assay results with GST-YY1 WT or S118A with CK2α, as described for panel A.