Fig 3.
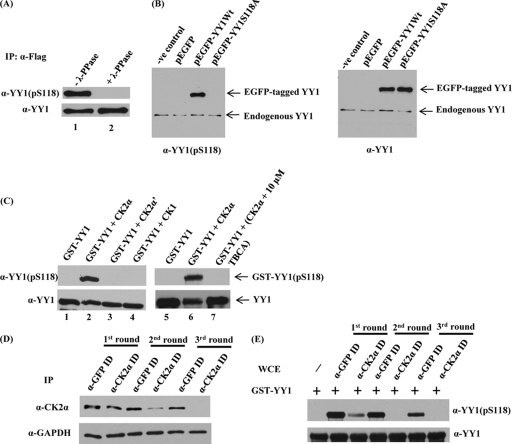
YY1 is phosphorylated in vivo at S118 and by CK2α in vitro, and immunodepletion of CK2α abolishes S118 phosphorylation in vitro. (A) Flag-YY1 was immunoprecipitated from a HeLa cell line stably overexpressing Flag-YY1 by using anti-Flag mouse MAb cross-linked to resin beads. Immune complexes bound to the beads were washed with lysis buffer, resuspended in phosphatase buffer, and incubated at 30°C, with (lane 2) or without (lane 1) λ-phosphatase for 30 min. Samples were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was examined for YY1 phosphorylated at serine 118 by using anti-YY1 (pS118) antibody. The blot was stripped and hybridized with anti-YY1 (H10) to verify equal amounts of Flag-YY1. (B) pEGFP-YY1 (WT), the nonphosphorylatable mutant pEGFP-YY1 (S118A), empty pEGFP vector, and the transfection control were expressed transiently in HeLa cells. At 24 h posttransfection, WCEs were prepared, separated by SDS-PAGE, and transferred to a membrane for analysis with anti-YY1 (pS118) antibody and anti-YY1 (H10). (C) Cold in vitro kinase assay of GST-YY1 (WT) with CK2α, CK2α′, or CK1. A CK2-specific inhibitor, TBCA, was added to the kinase reaction mixture (lane 7) at the indicated concentration. All lanes contained purified CK2α as identified, except for lane 1 and lane 5, which served as negative controls. Kinase reaction mixtures were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were examined first for YY1 phosphorylation at serine 118 by using anti-YY1 (pS118) antibody. GST-YY1 loading was examined by stripping and hybridizing the blot with anti-YY1 (H10). (D) Whole HEK293 cell extracts were subjected to three sequential rounds of immunodepletion (ID) with GFP antibody as a control or antibody to the α-catalytic subunit of CK2. Following ID, CK2α expression levels were assessed by separating cell lysates by SDS-PAGE and analyzing by Western blotting using anti-CK2α antibody. Also, the blot was stripped and hybridized with anti-GAPDH as a loading control. (E) Western blot analysis was performed after cold in vitro kinase assay reactions with both the control and CK2α-immunodepleted HEK293 WCEs from all the immunodepletion rounds, as the sources for kinase activity, and bacterially expressed GST-YY1 bound to glutathione beads as substrate. Reaction mixtures were separated on SDS-PAGE, transferred to nitrocellulose membranes, probed with anti-YY1 (pS118), and then stripped and reprobed with anti-YY1 (H10) antibody.