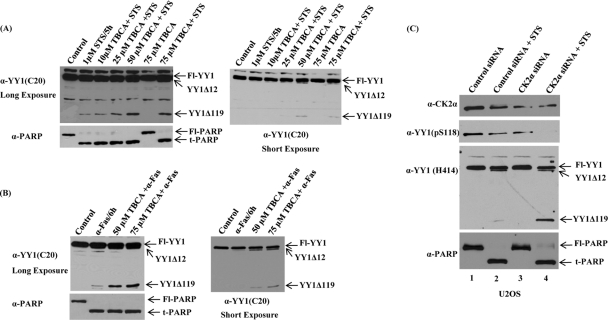
Fig 6.
CK2α knockdown in vivo enhances YY1 cleavage in the presence of apoptotic stimuli. (A and B) Asynchronous HeLa cells were preincubated with TBCA (a specific CK2 inhibitor), or with DMSO as a control, for 24 h at the indicated concentrations. After 24 h, either STS (A) or anti-Fas (B) anitbody was added to cells at 1 μM for 5 h or 100 ng/ml for 6 h, respectively, in the absence or presence of TBCA. WCEs were prepared, and proteins were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Cleavage of full-length and endogenous YY1 was detected by hybridizing the blot with anti-YY1 antibody (C20; rabbit polyclonal). Two exposures of the blot showed the apoptotic YY1Δ119 and YY1Δ12 cleavage products. The blots were stripped and reprobed by Western analysis using anti-PARP antibody. (C) U2OS cells were transiently transfected with control siRNA or siRNA to knock down CK2α. Following siRNA transfections for 48 h, cells were either left untreated or treated with the apoptotic stimulus STS at 1 μM for 5 h. CK2α expression levels, as well as the phosphorylation status of S118 on YY1, were assessed by separating cell lysates by SDS-PAGE and analyzing by Western blotting using anti-CK2α antibody and anti-YY1 (pS118) antibody, respectively. Also, the same samples were analyzed with anti-YY1 (H414) antibody (to detect endogenous YY1 protein levels and cleavage) and anti-PARP antibody (a marker of apoptosis).