Abstract
A pool of selected lactic acid bacteria was used for the sourdough fermentation of various cereal flours with the aim of synthesizing antioxidant peptides. The radical-scavenging activity of water/salt-soluble extracts (WSE) from sourdoughs was significantly (P < 0.05) higher than that of chemically acidified doughs. The highest activity was found for whole wheat, spelt, rye, and kamut sourdoughs. Almost the same results were found for the inhibition of linoleic acid autoxidation. WSE were subjected to reverse-phase fast protein liquid chromatography. Thirty-seven fractions were collected and assayed in vitro. The most active fractions were resistant to further hydrolysis by digestive enzymes. Twenty-five peptides of 8 to 57 amino acid residues were identified by nano-liquid chromatography-electrospray ionization-tandem mass spectrometry. Almost all of the sequences shared compositional features which are typical of antioxidant peptides. All of the purified fractions showed ex vivo antioxidant activity on mouse fibroblasts artificially subjected to oxidative stress. This study demonstrates the capacity of sourdough lactic acid bacteria to release peptides with antioxidant activity through the proteolysis of native cereal proteins.
INTRODUCTION
Interest in health-promoting functional foods, dietary supplements, and pharmaceutical preparations containing peptides deriving from food proteins is markedly increasing (24). Bioactive peptides are defined as specific protein fragments that have positive effects on body functions or conditions and that may influence human health (23). Usually, bioactive peptides correspond to cryptic sequences from native proteins, which are released mainly through hydrolysis by digestive, microbial, and plant proteolytic enzymes, and their levels generally increase during food fermentation (24). In vitro and in vivo studies show a large spectrum of biological functions attributed to bioactive peptides, such as opioid-like (19), mineral binding (9), immunomodulatory (15), antimicrobial (25), antioxidative (27), antithrombotic (46), hypocholesterolemic (53), and antihypertensive (21) activities. The release of various bioactive peptides (e.g., angiotensin I-converting enzyme [ACE]-inhibitory peptides) from milk proteins through proteolysis by lactic acid bacteria is the best documented (24). Recently, interest in antioxidant peptides derived from food proteins has increased, and evidence that bioactive peptides prevent oxidative stresses associated with numerous degenerative aging diseases (e.g., cancer and arteriosclerosis) is accumulating (2).
Overall, antioxidants have many applications in food industries. The delay of food discoloration and deterioration, which occur as a consequence of oxidative processes, markedly improves food preservation. The radical-mediated oxidation of fats and oils is one of the major causes of spoilage for lipid-containing foods during processing and storage (36). Antioxidants are extensively tested for the absence of carcinogenicity and other toxic effects in themselves, in their oxidized forms, and in the products of their reactions with food constituents; for their effectiveness at low concentrations; and for the absence of the ability to impart an unpleasant flavor to the food in which they are used (28). The use of antioxidants in food products is governed by regulatory laws of individual countries or by internal standards (28). Even though many synthetic compounds have antioxidant properties, only a few of them have been accepted as GRAS (generally recognized as safe) for use in food products by international bodies such as the Joint FAO/WHO Expert Committee on Food Additives and the European Community's Scientific Committee for Food. Toxicological studies are crucial in determining the safety of an antioxidant and also in determining the acceptable daily intake (ADI) levels (28). ADIs for widely used antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and gallates, have changed over the years mainly because of their toxicological effects in various species (20, 28, 33, 50), and new toxicological data for some of the synthetic antioxidants cautioned against their use (28).
Natural antioxidants recently attracted the attention of many food manufacturers as a result of the desire to produce healthy foods (28). Biologically active peptides with potential antioxidant activity have been derived from many animal and plant protein sources (35, 43, 49). They were already isolated from peanut kernels, rice bran, sunflower protein, alfalfa leaf protein, corn gluten meal, frog skin, yam, egg yolk protein, milk kefir, soymilk kefir, medicinal mushrooms, mackerel, curry leaves, cotton leafworm, casein, algal protein waste, wheat gluten, and buckwheat protein (43). It was argued that antioxidant peptides act as inhibitors of lipid peroxidation, as direct scavengers of free radicals, and/or as agents to chelate transition metal ions that catalyze the generation of radical species (43). Antioxidant peptides usually are constituted by 2 to 20 amino acidic residues and have molecular masses of less than 6.0 kDa (26, 48). The antioxidant activity seems to be strongly correlated with amino acid composition, conformation, and hydrophobicity (5).
Cereals are staple foods in the human diet. They are considered one of the most important sources of dietary carbohydrates, proteins, vitamins, minerals, and fibers for people all over the world. Large proportions of cereals are processed into foods and beverages by fermentation (e.g., sourdough) prior to consumption. Although biological activities such as ACE inhibition and antimicrobial and anticancer activities were reported for cereal peptides (7, 8, 38, 40, 41), to the best of our knowledge only a few studies (43) have investigated the potential release of antioxidant peptides during cereal fermentation.
This study aimed at investigating the in vitro and ex vivo antioxidant potential of different cereal flours, which were subjected to sourdough fermentation by selected lactic acid bacteria. Bioactive peptides were purified and identified to determine structural relationships with antioxidant properties.
MATERIALS AND METHODS
Microorganisms.
Lactobacillus alimentarius 15 M, Lactobacillus brevis 14G, Lactobacillus sanfranciscensis 7A, and Lactobacillus hilgardii 51B were previously selected based on their capacity to hydrolyze gliadins (14). L. sanfranciscensis LS3, LS10, LS19, LS23, LS38, and LS47 were selected based on their peptidase systems, with particular reference to activities toward Pro-rich peptides (12). The strains were used in mixture for sourdough fermentation. Lactobacilli were propagated for 24 h at 30°C in MRS broth (Oxoid, Basingstoke, Hampshire, England), with the addition of fresh yeast extract (5%, vol/vol) and 28 mM maltose, at a final pH of 5.6 (modified MRS [mMRS]). When used for sourdough fermentations, lactic acid bacterial cells were cultivated until the late exponential phase of growth was reached (ca. 10 h), washed twice in 50 mM phosphate buffer, pH 7.0, and resuspended in tap water (total bacteria count, <100 CFU/ml), which was used for making the dough. The enumeration of lactic acid bacteria was carried out by plating serial dilutions of sourdoughs on mMRS agar medium (Oxoid) at 30°C for 48 h.
Sourdough fermentation.
As determined by AACC official methods (1), the characteristics of the flours used in this study are reported in Table 1. Each flour was used to prepare a sourdough containing 120 g flour and 280 g of tap water, with a dough yield (DY; dough weight × 100/flour weight) of 330 (DY 330). Fermentation with the pool of selected lactic acid bacteria (initial cell density of 5 × 107 CFU/g of dough) was carried out at 37°C for 24 h under stirring conditions (ca. 200 rpm). Control doughs (DY 330) without bacterial inoculum were chemically acidified to pH 3.5 by a mixture of lactic and acetic acids (molar ratio, 4:1) and incubated under the same conditions.
Table 1.
Characteristics of the flours used for sourdough fermentation
| Flourb | Result by parametera |
|||||
|---|---|---|---|---|---|---|
| Moisture (%) | Carbohydrate (% of d.m.) | Protein (% of d.m.) | Lipid (% of d.m.) | Fiber (% of d.m.) | Ash (% of d.m.) | |
| Whole wheat | 12.3 ± 0.3 | 67.1 ± 1.1 | 11.0 ± 0.7 | 1.7 ± 0.1 | 9.6 ± 0.1 | 1.2 ± 0.1 |
| Durum wheat | 12.5 ± 0.5 | 63.2 ± 1.0 | 12.9 ± 0.8 | 2.8 ± 0.2 | 8.8 ± 0.5 | 1.5 ± 0.1 |
| Rye | 9.8 ± 0.3 | 77.5 ± 1.5 | 9.4 ± 0.6 | 1.7 ± 0.1 | 14.6 ± 0.6 | 1.5 ± 0.1 |
| Spelt | 10.4 ± 0.1 | 67.1 ± 1.2 | 15.1 ± 0.3 | 2.5 ± 0.1 | 6.8 ± 0.3 | 2.5 ± 0.2 |
| Oat | 12.0 ± 0.3 | 62 ± 0.8 | 12.6 ± 0.8 | 12.3 ± 0.6 | 11.4 ± 0.6 | 1.9 ± 0.1 |
| Rice | 11.9 ± 0.3 | 80.1 ± 1.2 | 5.9 ± 0.9 | 1.4 ± 0.1 | 2.4 ± 0.2 | 0.6 ± 0.2 |
| Kamut | 11.7 ± 0.2 | 69.2 ± 0.5 | 13.5 ± 0.3 | 2.1 ± 0.2 | 8.3 ± 0.1 | 0.9 ± 0.3 |
| Barley | 12.1 ± 0.1 | 70.5 ± 0.4 | 10.5 ± 0.1 | 1.6 ± 0.2 | 9.1 ± 0.2 | 0.9 ± 0.1 |
| Maize | 12.5 ± 0.4 | 76.8 ± 0.8 | 8.7 ± 0.1 | 2.7 ± 0.2 | 2.1 ± 0.3 | 0.6 ± 0.3 |
d.m., dry matter. Mean values ± standard deviations are reported.
For each flour, three samples were analyzed twice.
WSE.
Water/salt-soluble extracts (WSE) were prepared from each dough according to the method originally described by Osborne (32) and further modified by Weiss et al. (51). An aliquot of each dough (containing 7.5 g of flour) was diluted with 30 ml of 50 mM Tris-HCl (pH 8.8), held at 4°C for 1 h, vortexed at 15-min intervals, and centrifuged at 20,000 × g for 20 min. The supernatants, containing the water/salt-soluble nitrogen fraction, were used for in vitro assays of the antioxidant activity. The concentrations of peptides in the WSE and purified fractions were determined by the o-phtaldialdehyde (OPA) method (6). A standard curve prepared using tryptone (0.25 to 1.5 mg/ml) was used as the reference. The use of peptone gave a similar standard curve.
DPPH radical-scavenging activity.
The scavenging effect of WSE and purified fractions on 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals was measured according to the method of Shimada et al. (45), with some modifications (52). Freeze-dried samples were dissolved in 0.1 M phosphate buffer (pH 7.0) at a peptide concentration of 1 mg/ml, and 2 ml of each solution was added to 2 ml of 0.1 mM DPPH dissolved in 95% ethanol. The mixture was shaken and left for 30 min at room temperature, and the absorbance of the resulting solution was read at 517 nm. The absorbance measured after 10 min was used for the calculation of the DPPH radicals scavenged by WSE or purified peptide fractions (39). A lower absorbance represents a higher DPPH radical-scavenging activity. The scavenging effect was expressed as shown in the following equation: DPPH radical-scavenging activity (%) = [(blank absorbance − sample absorbance)/blank absorbance] × 100. Butylated hydroxytoluene (BHT) and α-tocopherol (1 mg/ml) also were assayed as antioxidant references.
Inhibition of linoleic acid autoxidation.
The antioxidant activity of WSE and purified fractions also was measured according to the method of Osawa and Namiki (31), with some modifications. After freeze-drying, 1.0 mg of each sample was suspended in 1.0 ml of 0.1 M phosphate buffer (pH 7.0) and added to 1 ml of linoleic acid (50 mM), which was previously dissolved in ethanol (99.5%). Incubation in a glass test tube, tightly sealed with a silicon rubber cap, was allowed at 60°C in the dark for 8 days. The degree of oxidation was determined by measuring the values of ferric thiocyanate according to the method described by Mitsuta et al. (30). One hundred microliters of the sample was mixed with 4.7 ml of 75% (vol/vol) ethanol, 0.1 ml of 30% (wt/vol) ammonium thiocyanate, and 0.1 ml of 0.02 M ferrous chloride, which had been dissolved in 1 M HCl. After 3 min, the degree of color development, which represents the oxidation of linoleic acid, was measured spectrophotometrically at 500 nm. BHT and α-tocopherol (1 mg/ml) also were assayed as antioxidant references. A negative control (without antioxidants) also was considered.
Purification of antioxidant peptides.
WSE were fractionated by ultrafiltration (Ultrafree-MC centrifugal filter units; Millipore) by using three different membrane sizes with 50-, 30-, and 10-kDa cutoffs (fractions A, B, and C, respectively). Aliquots of 400 μl of WSE were centrifuged at 10,000 × g for 60 min. After ultrafiltration, fractions were used for the DPPH radical-scavenging activity assay. The 10-kDa partially purified fractions (C) were further fractionated (37 fractions) by reverse-phase fast performance liquid chromatography (RP-FPLC) using a Resource RPC column and ÄKTA FPLC equipment with the UV detector operating at 214 nm (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Aliquots containing 1 mg/ml of peptides were added to 0.05% (vol/vol) trifluoroacetic acid (TFA) and centrifuged at 10,000 × g for 10 min. The supernatant was filtered with a 0.22-μm-pore-size filter and loaded onto the column. Gradient elution was performed at a flow rate of 1 ml/min using a mobile phase composed of water and acetonitrile (CH3CN) containing 0.05% TFA. The concentration of CH3CN was increased linearly from 5 to 46% between 16 and 62 min and from 46 to 100% between 62 and 72 min. Solvents were removed from collected fractions by freeze-drying. The fractions were redissolved in sterile water and subjected to in vitro assays for antioxidant activity.
Proteolysis and heat stability of purified fractions.
The purified fractions from WSE, which showed the highest antioxidant activities, were subjected to sequential protein hydrolysis by digestive enzymes according to the method described by Pasini et al. (34). Briefly, freeze-dried aliquots corresponding to 10 mg of peptides were suspended in 400 μl of 0.2 N HCl (pH 2.0) containing 0.05 mg/ml of pepsin (EC 3.4.23.1) (Sigma-Aldrich CO., St. Louis, MO) and homogenized in a Sterilmixer Lab (PBI International). After 30 min of incubation at 37°C under stirring conditions (150 rpm), 115 μl of a solution of 1 M boric acid and 0.5 N NaOH, adjusted to pH 6.8 with 5 N HCl and containing 0.25 mg/ml of pancreatin (Sigma) and 0.0087 mg/ml of trypsin (EC 3.4.21.4) (Sigma), was added. The resulting pH was 7.6. Pancreatic digestion lasted 150 min. Digested samples were heated for 5 min at 100°C and centrifuged at 12,000 × g for 20 min to recover the supernatants. After treatments, samples were subjected to in vitro assays for antioxidant activity.
Identification of antioxidant peptides.
The fractions of WSE with the highest radical-scavenging activities were subjected to a second step of purification through RP-HPLC under the conditions described previously and using an ÄKTA purifier apparatus (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). The centers of the peaks were collected, freeze-dried, and used for mass spectrometry (MS) analysis.
The identification of peptides was carried out by nano-liquid chromatography-electrospray ionization-tandem MS (nano-LC-ESI-MS/MS) using a Finningan LCQ Deca XP Max ion trap mass spectrometer (ThermoElectron) through the nano-ESI interface. According to the manufacturer's instrument settings for nano-LC-ESI-MS/MS analyses, MS/MS spectra were automatically taken by Xcalibur software (ThermoElectron) in the positive ion mode. Spectra were processed using the software BioWorks 3.2 (ThermoElectron), generating peak lists suitable for database searches. Peptides were identified using an MS/MS ion search with the Mascot search engine (Matrix Science, London, England) and of the NCBInr protein database (National Center for Biotechnology Information, Bethesda, MD). For the identification of peptides, the following parameters were considered: enzyme, none; instrument type, ESI-trap; peptide mass tolerance, ± 0.1%; and fragment mass tolerance, ± 0.5 Da. Results from peptide identification were subjected to a manual evaluation as described by Chen et al. (5), and the validated peptide sequences explained all of the major peaks in the MS/MS spectra.
Effect of purified peptides on viability of oxidation-induced cells.
Mouse fibroblasts (Balb 3T3; clone A31; ATCC CCL-163TM) were cultured under a humidified atmosphere (5% CO2, 37°C) using Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (wt/vol) fetal bovine serum (FBS), 1 mM glutamine, and 100 μg/ml penicillin-streptomycin. The culture medium was renewed every 2 days, and after four passages the cultures were used to determine cell viability. Cell viability was measured using the MTT [3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide] method (18), and the capacity of succinate dehydrogenase to convert 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into visible formazan crystals was assessed. For the MTT assay, cells were seeded in a 96-well plate at a density of 5× 104 cells/well and incubated for 16 h. Cells subsequently were treated with the antioxidant-purified fractions and incubated for a further 16 h. The final concentrations of peptides in the reaction mixture were 1.20, 0.81, 2.33, 0.41, 1.79, 2.88, 1.69, and 1.67 mg/ml for WSE from fraction 3 of whole wheat, fractions 2 and 3 of spelt, fractions 5 and 36 of rye, and fractions 2, 3, and 37 of kamut, respectively. A negative control, without the addition of peptide fractions, was used. α-Tocopherol (250 and 500 μM) was used as the positive control. Following the removal of FBS, cells were exposed to 150 μM hydroxide peroxide for 2 h. For each well, 250 μl of MTT (0.5 mg/ml final concentration) dissolved in DMEM was added, and incubation (37°C) in the dark was allowed for 1 h. Finally, dimethylsulfoxide (DMSO) (250 μl) was added to solubilize the formazan which was formed. The level of formazan was determined by measuring the optical density at 570 nm with a Biotek EL808 microplate reader (Winooski, VT). The relative cell viability was determined as the level of MTT converted into formazan salt. Data were expressed as the mean percentage of viable cells compared to that of the control culture before oxidative stress.
Statistical analysis.
Data were subjected to one-way analysis of variance (ANOVA); the pair comparison of treatment means was achieved by Tukey's procedure at P < 0.05 using Statistica for Windows.
RESULTS
Sourdough fermentation.
After 24 h of fermentation at 37°C, lactic acid bacteria reached cell densities ranging from 1.2 ± 0.4 to 6.2 ± 0.5 × 109 CFU/g. The lowest values were found during the sourdough fermentation of oat, rice, and maize flours. No significant (P > 0.05) differences were found among cell densities of sourdoughs made with whole wheat, durum wheat, rye, spelt, kamut, and barley flours. Before fermentation, the pHs were 5.20 ± 0.05, 5.27 ± 0.06, 5.82 ± 0.04, 5.45 ± 0.08, 4.56 ± 0.02, 5.15 ± 0.02, 5.37 ± 0.03, 5.23 ± 0.03, and 4.45 ± 0.02 for whole wheat, durum wheat, rye, spelt, oat, rice, kamut, barley, and maize doughs, respectively. After fermentation, the pHs ranged from 3.40 ± 0.03 to 3.88 ± 0.05. The lowest pHs were found for rice (3.26 ± 0.02) and whole wheat (3.40 ± 0.03) sourdoughs. The highest value was found for oat sourdough (3.88 ± 0.05).
In vitro antioxidant activity of WSE.
WSE from sourdoughs and control doughs had pHs ranging from 6.5 ± 0.2 to 7.0 ± 0.2 as a consequence of extraction with the Tris-HCl buffer. As determined by the OPA method, the concentrations of peptides of WSE were 0.68 ± 0.04, 0.41 ± 0.02, 1.12 ± 0.05, 0.68 ± 0.05, 0.31 ± 0.01, 0.18 ± 0.02, 0.61 ± 0.03, 0.71 ± 0.03, and 0.91 ± 0.04 mg/ml for whole wheat, durum wheat, rye, spelt, oat, rice, kamut, barley, and maize sourdoughs, respectively. The concentrations of peptides of WSE from the chemically acidified doughs was ca. 2 to 3 times lower than those of the respective sourdoughs.
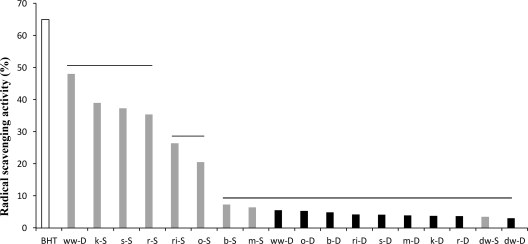
During the radical-scavenging assay, the colored stable DPPH radical is reduced to DPPH-H (nonradical form) in the presence of an antioxidant or a hydrogen donor. The DPPH radical without antioxidants was stable over time. Under the assay conditions, 100% of activity corresponds to the complete scavenging of DPPH radicals (50 μM final concentration) after 10 min of incubation with the antioxidant compounds. According to previous studies (38, 50), the color intensity of the DPPH radical showed a logarithmic decline in the presence of BHT. The radical-scavenging activity toward the stable radical DPPH was in the range of 3.0% ± 0.2% to 5.5% ± 0.2% for WSE chemically acidified doughs (Fig. 1). No significant (P > 0.05) differences were found between samples. BHT (1 mg/ml) showed an activity of ca. 65% (10 min). Almost all WSE from sourdoughs showed a marked increase of the radical-scavenging activity compared to that of the chemically acidified doughs. Durum wheat, maize, and barley sourdoughs did not show appreciable increases. The highest scavenging activity was found for whole wheat (48.0% ± 0.4%), kamut (39.0% ± 0.2%), spelt (37.3% ± 0.4%) and rye (35.4% ± 0.5%) sourdoughs. Based on these results, WSE from chemically acidified doughs were not further characterized.
Fig 1.
DPPH radical-scavenging activity of the WSE from chemically acidified doughs (black bars; -D) and sourdoughs (grey bars; -S) fermented with the pool of selected lactic acid bacteria. BHT (white bar) (1 mg/ml) was used as the positive control. ww, whole wheat; dw, durum wheat; r, rye; s, spelt; o, oat; ri, rice; k, kamut; b, barley; m, maize. Data are the means from three independent experiments. Means connected by the same horizontal line are similar by the Tukey's comparison test (P > 0.05).
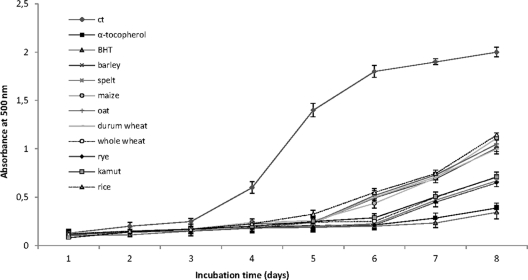
Lipid peroxidation is thought to proceed via the radical-mediated abstraction of hydrogen atoms from methylene carbons in polyunsaturated fatty acids (35). The absorbance of WSE at 500 nm from sourdoughs was higher than that of the positive controls, thus showing a lower inhibition of linoleic acid autoxidation (Fig. 2). In agreement with the previous findings for radical-scavenging activities, the oxidation of linoleic acid was markedly inhibited by the addition of WSE from whole wheat, spelt, rye, and kamut sourdoughs. WSE from durum wheat, rice, oat, barley, and maize sourdoughs showed weak activities.
Fig 2.
Lipid peroxidation inhibitory activity of the WSE from sourdoughs fermented with the pool of selected lactic acid bacteria. The activity was measured under a linoleic acid oxidation system for 8 days. BHT and α-tocopherol (1 mg/ml) were used as the positive controls. A negative control, without antioxidants, also was considered (ct). Data are the means from three independent experiments. Bars represent standard deviations.
Aiming at the subsequent identification of antioxidant peptides, WSE of whole wheat, spelt, rye, and kamut sourdoughs were subjected to ultrafiltration (cutoffs of 10, 30, and 50 kDa) and further assayed for radical-scavenging activity. All fractions from ultrafiltration showed activity toward the DPPH radical, suggesting that the molecular mass of antioxidant compounds was less than 10 kDa (data not shown).
Purification and characterization of antioxidant peptide fractions.
Thirty-seven fractions were collected by the RP-FPLC separation of each WSE (aliquots contained 10 mg of peptides, as determined by the OPA method). The peptide profiles of the different WSE were similar (Fig. 3).
Fig 3.
RP-FPLC chromatograms of WSE of whole wheat (A), spelt (B), rye (C), and kamut (D) sourdoughs fermented with the pool of selected lactic acid bacteria. The dashed lines refer to the percentages of radical scavenging activity (line of dashes and dots) and to the gradient of eluent B (line of dashes only). Arrows indicate peptide fractions with the highest antioxidant activity. ct, control.
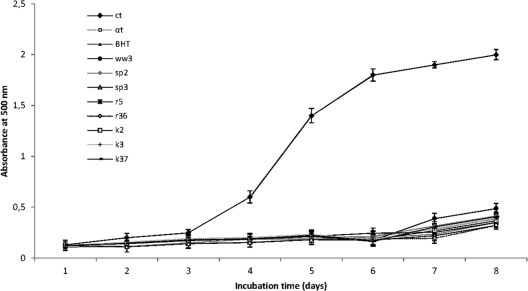
Collected fractions were freeze-dried, dissolved in ca. 600 μl of distilled water, and assayed for DPPH radical-scavenging activity and the inhibition of linoleic acid autoxidation. Based on the DPPH radical-scavenging assay, eight fractions with the highest levels of activity were selected (Fig. 3). They were fraction 3 (activity of ca. 27%, peptide concentration of 1.20 ± 0.04 mg/ml) from whole wheat; fractions 2 (ca. 90%, 0.81 ± 0.03 mg/ml) and 3 (ca. 75%, 2.33 ± 0.04 mg/ml) from spelt; fractions 5 (ca. 45%, 0.41 ± 0.02 mg/ml) and 36 (ca. 38%, 1.79 ± 0.05 mg/ml) from rye; and fractions 2 (ca. 48%, 2.88 ± 0.03 mg/ml), 3 (ca. 49%, 1.69 ± 0.05 mg/ml), and 37 (ca. 36%, 1.67 ± 0.04 mg/ml) from kamut. All of the fractions showed inhibitions of linoleic peroxidation similar to those of α-tocopherol (80.6%) and BHT (82.3%) (Fig. 4). In particular, fraction 3 from spelt and fraction 36 from rye showed the highest activity (83.9%). No statistical correlation was found between the concentration of peptides and the antioxidant activities.
Fig 4.
Lipid peroxidation inhibitory activity of the purified peptide fractions of the WSE from sourdoughs fermented with the pool of selected lactic acid bacteria. The activity was measured in a linoleic acid oxidation system in the dark for 8 days. BHT and α-tocopherol (1 mg/ml) were used as the positive controls. ct, negative control (without antioxidants); αt, α-tocopherol; ww3, fraction 3 of whole wheat; sp2 and sp3, fractions 2 and 3 of spelt, respectively; r5 and r36, fractions 5 and 36 of rye, respectively; and k2, k3, and k36, fractions 2, 3, and 37 of kamut, respectively. Data are the means from three independent experiments. Bars represent standard deviations.
The purified fractions were subjected to sequential hydrolysis by pepsin, trypsin, and pancreatin, which mimicked the digestive process. As determined by the free radical-scavenging assay, fraction 37 from kamut and fractions 5 and 36 from rye showed a less than 10% decrease in antioxidant activity compared to that of undigested fractions (35, 42, and 35% of activity, respectively). No significant (P > 0.05) decrease was found for the other fractions (the antioxidant activity was 27% for fraction 3 from whole wheat, 89 and 76% for fractions 2 and 3 from spelt, respectively, and 49 and 49% for fractions 2 and 3 from kamut, respectively). The antioxidant activity of the digested peptide fractions was not affected by heating for 5 min at 100°C.
Isolation and identification of antioxidant peptides.
Twenty-five peptides of 8 to 57 amino acid residues and molecular masses varying from 769.8 to 5,338.5 Da were identified by nano-LC-ESI-MS/MS analysis (Table 2). All of the peptides were found in the NCBInr database as being encrypted in different cereal proteins (accession numbers are reported in Table 2).
Table 2.
Sequences of peptides contained in the purified fractions of WSE of whole wheat, spelt, rye, and kamut sourdoughs fermented with the pool of selected lactic acid bacteria
| Fraction (fraction no.) and peptide no. | Sequencea | Score | Charge | Mass |
Source protein NCBI accession no. and description | ||
|---|---|---|---|---|---|---|---|
| Calculated | Expected | Change | |||||
| Whole wheat (3) | |||||||
| 1 | MAPAAVAAAEAGSK | 17 | 2 | 1,243.6230 | 1,243.0196 | −0.6034 | GH32_ORYSJ; probable indole-3-acetic acid-amidosynthetase; P0C0M2 |
| 2 | DNIPIVIR | 14 | 2 | 938.5549 | 937.5898 | −0.9651 | AKH2_MAIZE; bifunctional aspartokinase/homoserine dehydrogenase 2; P49080 |
| Spelt (2) | |||||||
| 3 | AIAGAGVLSGYDQLQILFFGK | 13 | 3 | 2,167.1677 | 2,166.0752 | −1.0925 | ADT1_MAIZE; ADP, ATP carrier protein 1, mitochondrial; P04709 |
| 4 | GNQEKVLELVQR | 18 | 2 | 1,411.7783 | 1,412.4122 | 0.6339 | FH16_ORYSJ; formin-like protein 16 |
| 5 | PAGSAAGAAP | 14 | 2 | 769.8311 | 769.4197 | −0.4114 | C3H31_ORYSJ; zinc finger CCH domain-containing protein 31; Q7XPK1 |
| 6 | EALEAMFL | 12 | 3 | 924.1021 | 923.5322 | −0.5699 | IAA31_ORYSJ; auxin-responsive protein IAA31; P0C133 |
| Spelt (3) | |||||||
| 7 | AAGAAAAARSAGQCGR | 16 | 3 | 1,387.6738 | 1,388.3942 | 0.7204 | SAP17_ORYSJ; zinc finger AN1 domain-containing stress-associated protein 17; Q6H595 |
| 8 | ITFAAYRR | 12 | 2 | 998.1621 | 997.6323 | −0.5298 | NIP41_ORYSJ; aquaporin NIP4-1; Q9ASI1 |
| 9 | HPVPPKKK | 14 | 3 | 912.2177 | 912.5011 | 0.2834 | CCF11_ORYSJ; putative cyclin-F1-1; Q6K1Z1 |
| Rye (5) | |||||||
| 10 | VFVDEGLEVLGWRPVPFNVSVVGRNAK | 18 | 2 | 2,982.6080 | 2,983.0406 | 0.4326 | GLTB_MAIZE; ferredoxin-dependent glutamate synthase, chloroplastic; P23225 |
| 11 | RLSLPAGAPVTVAVSP | 14 | 2 | 1,535.8101 | 1,534.9327 | −0.8774 | CSLD4_ORYSJ; cellulose synthase-like protein d4 Oryza sativa subsp. japonica; Q2QNS6 |
| 12 | NANGELCPNNMCCSQWGYCGLGSEFCGNGCQSGACCPEK | 13 | 3 | 4,033.4843 | 4,032.5905 | −0.8938 | AGI_ORYSJ; lectin; Q0JF21 |
| 13 | LCPVHRAADL | 14 | 2 | 1,095.3231 | 1,094.6324 | −0.6907 | CSLD4_ORYSJ; cellulose synthase-like protein d4; Q2QNS6 |
| Rye (36) | |||||||
| 14 | PAEMVAAALDR | 12 | 2 | 1,484.7511 | 1,483.8343 | −0.9168 | SLY1_OTYSJ; SEC1 family transport protein SLY1; Q851W1 |
| 15 | KVALMSAGSMH | 14 | 3 | 1,131.2679 | 1,131.3802 | 0.1123 | MLOH1_HORVU; MLO protein homolog 1; O49873 |
| 16 | DLADIPQQQRLMAGLALVVATVIFLK | 13 | 3 | 2,822.6092 | 2,822.9826 | 0.3734 | CP51_SORBI; obtusifoliol 14-alpha demethylase; P93846 |
| 17 | KNGSIFNSPSATAATIIHGHNYSGLAYLDFVTSK | 13 | 2 | 3,580.7950 | 3,581.6329 | 0.8379 | KSL6_ORYSJ; Ent-isokaur-15-ene synthase; A4KAG8 |
| 18 | GTIFFSQEGDGPTSVTGSVSGLKPGLHGFHVHALGDTTNGCMSTGPHFNPTGK | 12 | 2 | 5,338.5201 | 5,338.2306 | −0.2895 | SODC2_ORYSJ; superoxide dismutase (Cu-Zn); P28757 |
| Kamut (2) | |||||||
| 19 | YEWEPTVPNFDVAKDVTDM | 13 | 2 | 2,255.0093 | 2,254.3380 | −0.6713 | KRP3_ORYSJ; cyclin-dependent kinase inhibitor 3; Q2R185 |
| 20 | GVSNAAVVAGGH | 12 | 3 | 1,037.5254 | 1,038.6974 | 1.1720 | SUT5_ORYSI; sucrose transport protein SUT5; A2X6E6 |
| Kamut (3) | |||||||
| 21 | DAQEFKR | 13 | 2 | 892.4403 | 891.7372 | −0.7031 | HFN40_MAIZE; suppressor protein HFN40; P82865 |
| 22 | PPGPGPGPPPPPGAAGRGGGG | 14 | 3 | 1,704.8721 | 1,703.9566 | −0.9155 | FH18_ORYSJ; formin-like protein 18; Q6MWG9 |
| 23 | HKEMQAIFDVYIMFIN | 14 | 1 | 2,000.3734 | 1,999.0342 | −1.3392 | ZRP4_MAIZE; O-methyltransferase ZRP4; P47917 |
| Kamut (37) | |||||||
| 24 | TGGGSTSSSSSSSSSLGGGASRGSVVEAAPPATQGAAAANAPAVPVVVVDTQEAGIR | 15 | 3 | 5,124.5196 | 5,124.3480 | −0.1715 | SLR1_ORYSJ; DELLA protein SLR1; Q7G7J6 |
| 25 | DTAAGYVAPPDPAVSTGDYGLAGAEAPHPHESAVMSGAAAAAVAPGGEAYTR | 15 | 3 | 4,921.2889 | 4,920.3312 | −0.9577 | DHR25_ORYSJ; dehydrin Rab25; P30287 |
Single-letter amino acid codes are used.
Two peptides of 14 and 8 amino acid residues, with hydrophobic ratios of 64 and 50% (sequences n.1 and n.2, respectively), respectively, and a total net charge of 0 were identified from fraction 3 of whole wheat sourdough. Four and three peptides having 8 to 21 amino acid residues were identified from fractions 2 and 3 of spelt sourdough, respectively. Except for the sequences n.4 and n.9 (Table 2), the hydrophobic ratio was higher than 50%. Except for the sequence n.6, all peptides have net positive or neutral total charges. Four and five different peptides were identified from fractions 5 and 36 of rye sourdough, respectively. The major part of the sequences contained 26 to 53 amino acid residues. The sequences n.11, n.13, n.14, and n.15 were shorter (10 to 16 amino acid residues). Except for those of sequences n.12, n.17, and n.18, the hydrophobic ratio was greater than 48%. The total net charge of sequences n.18 and n.20 was negative, while it was positive or neutral for all of the other sequences. Two peptides from fractions 2 and 37 and three peptides from fraction 3 of kamut sourdough were identified. Sequences n.24 and n.25 of fraction 37 had the highest number of amino acid residues (57 and 52, respectively). The other sequences were shorter (fewer than 21 amino acid residues). Except for the sequence n.22, their hydrophobic ratios were higher than 35%. All of the peptides identified from fractions 2 and 3 had net positive or neutral charges, while sequences n.24 and n.25 from fraction 37 had net negative charges.
Effect of purified peptides on viability of oxidation-induced cells.
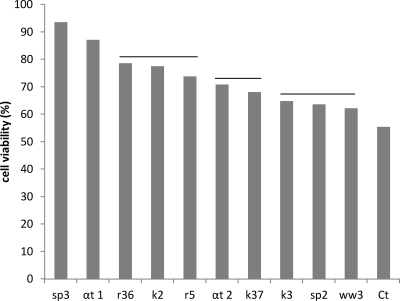
To investigate the capacity of the purified peptides to act as radical scavengers, cultured mouse fibroblasts were grown in the presence of eight purified fractions from whole wheat, spelt, rye, and kamut sourdoughs. Cells then were treated with hydroxide peroxide. Under the experimental conditions, cell viability was evaluated by assaying the capacity of functional mitochondria to catalyze the reduction of MTT to formazan salt via mitochondrial dehydrogenases. α-Tocopherol and all purified fractions increased cell survival compared to that of the negative control (55.4% ± 0.2% of cell viability after oxidative stress) (Fig. 5). In particular, purified fraction 3 of spelt sourdough showed a significantly (P < 0.05) higher activity than that induced by 500 μM α-tocopherol (93.6% ± 0.2% versus 87.2% ± 0.1%). Three other purified fractions (2 of kamut and 5 and 36 of rye sourdoughs) induced cell viability higher than that induced by 250 μM α-tocopherol (77.6% ± 0.1%, 73.9% ± 0.4%, 78.6% ± 0.4%, and 70.8% ± 0.3%, respectively).
Fig 5.
Effect of purified peptide fractions on cell viability of mouse fibroblasts. Mouse fibroblasts were cultured in Dulbecco's modified Eagle medium (DMEM) without fetal bovine serum and were incubated with purified fractions for 16 h. Oxidative stress was artificially induced by incubating cultured cells with 150 μM hydroxide peroxide for 2 h. The percentage of viable cells with respect to untreated cultures was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Ct, control without antioxidants; αt1 and αt2, α-tocopherol at 500 and 250 μg/liter, respectively; ww3, fraction 3 of whole wheat; sp2 and sp3, fractions 2 and 3 of spelt, respectively; r5 and r36, fractions 5 and 36 of rye, respectively; and k2, k3, and k36, fractions 2, 3, and 37 of kamut, respectively. Data are the means from three independent experiments analyzed twice. Means connected by the same horizontal line are similar by the Tukey's comparison test (P > 0.05).
DISCUSSION
Sourdough fermentation has a well-known role in improving the nutritional properties of wheat, rye, and oat baked goods (22). It stabilizes or increases the levels of various bioactive compounds, retards starch bioavailability (thus decreasing the glycemic index), and increases mineral bioavailability (10, 13, 42). To our knowledge, this is the first study reporting the capacity of sourdough lactic acid bacteria to release peptides with antioxidant activity through the proteolysis of native cereal proteins. In the areas of human nutrition and biochemistry, natural antioxidants from food sources are studied largely because of their potential health benefits with few or no side effects. Low molecular mass, low cost, high activity, and easy absorption are the main features of antioxidant peptides. Although synthetic antioxidants are more effective, natural antioxidants have a simpler structure, higher stability, and nonhazardous immune reactions (43). It is presumed that bioactive peptides deriving from cereal proteins are not immunogenic/allergenic toward healthy people but are immunogenic/allergenic for subjects suffering from specific allergies, intolerance, and/or sensitivity to them.
Whole wheat, durum wheat, rye, spelt, oat, rice, kamut, barley, and maize are the most common cereal flours used for making fermented baked goods. Sourdough fermentation by selected lactic acid bacteria was allowed for a long time and under semiliquid conditions, which are indispensable to fully exploit microbial proteolysis (16, 38). Under these processing parameters, a moderate but not too extensive degradation of the flour proteins occurred (38). The pool of lactic acid bacteria used for sourdough fermentation included 10 strains, which were previously selected based on proteinase and peptidase activities toward wheat proteins (37). Proteinase activity and, especially, a large portfolio of peptidases are the prerequisites to release bioactive peptides during sourdough fermentation (11, 17). The hydrolyzing activities responsible for the degradation of cereal proteins are not widespread in sourdough lactic acid bacteria, and in general it is very rare that a unique microbial strain possesses all the necessary enzymes (13). Previously, the same pool of lactic acid bacteria was successfully used to degrade epitopes responsible for celiac disease (37) and to synthesize ACE-inhibitory peptides during the sourdough fermentation of wheat and rye flours (38).
Notwithstanding an activation of cereal-endogenous enzymes due to acidification (16), the antioxidant activity of chemically acidified doughs (controls) was very low. WSE from several sourdoughs, which had almost the same level of acidity as that of the controls, showed an elevated in vitro radical-scavenging activity and the inhibition of linoleic acid autoxidation. Sourdoughs from whole wheat, rye, spelt, and kamut had the highest activities, which, however, at the concentration assayed were lower than those of BHT and α-tocopherol.
Many cereals are closely related species, but many factors, such as the high level of polymorphism, the ratio between different protein fractions (solubility classes), the amino acid composition and sequence, and the molecular mass of the individual polypeptides, differentiate the technological, structural, nutritional, and functional properties of flours (44). Thus, differences of the functional properties of peptides deriving from different cereals were expected. Aiming at purifying antioxidant peptides, eight fractions (the concentration of peptides was lower than 3 mg/ml) from whole wheat, spelt, rye, and kamut sourdoughs were selected after RP-FPLC separation. Six fractions eluted in the early zone of the acetonitrile gradient. The other two were eluted at the end of the acetonitrile gradient. The in vitro antioxidant activity of some of these fractions (3 and 36 from spelt and rye, respectively) was higher than that of the synthetic antioxidants used as positive controls. As previously reported (29, 38), purified fractions can show higher bioactivity than the raw extract as the consequence of the higher concentration of the active compound compared to that of the other constituents of the matrix.
To be active, antioxidant peptides should have the capacity to overcome hydrolysis and modifications at the intestinal level and to reach their targets (43). Overall, the antioxidant activity of the purified fractions seemed not to be affected by sequential in vitro treatments with digestive enzymes. A slight decrease of the antioxidant activity was found only for fraction 37 of kamut sourdough and fractions 5 and 36 of rye sourdough, which contained peptides having the largest size. After the in vitro assays, the ex vivo antioxidant activity of the purified fractions was determined for mouse fibroblasts, which were artificially subjected to oxidative stress. As shown by MTT assays, all of the purified fractions exhibited a marked protective effect. In particular, fractions 3 of spelt sourdough and 36 of rye sourdough, which also showed the highest in vitro inhibition of linoleic acid autoxidation, had an effect on the survival of mouse fibroblasts comparable to that of α-tocopherol. However, a specific in vivo test should be carried out to evaluate the effect on human health.
Twenty-five peptides were found in the purified fractions by nano-LC-ESI-MS/MS analysis. None of these peptides was previously reported to be an antioxidant (43). A mixture of peptides was identified in all of the active fractions. Overall, it was hypothesized that the strongest antioxidant activity was ascribed to the synergic effect between peptides rather than to the individual activity of the single peptides (5). Almost all of the sequences showed features typical of well-known antioxidant peptides, such as low molecular mass (43). The presence of amino acids, such as Tyr (Y), Trp (W), Met (M), Lys (K), Pro (P), Cys(C), His (H), Val (V), Leu (L), and Ala (A), would be ascribed, for different reasons, to the antioxidant activity of peptides (42). Except for the sequences DNIPIVIR and GTIFFSQEGDGPTSVTGSVSGLKPGLHGFHVHALGDTTNGCMSTGPHFNPTGK, found in fractions 3 of whole wheat sourdough and 36 of rye sourdough, respectively, all of the other sequences are constituted totally (e.g., HPVPPKKK from fraction 3 of spelt sourdough) or mostly by the amino acids described above. Hydrophobic amino acids enhance the solubility of peptides in lipids, thus facilitating access to hydrophobic radical species and to hydrophobic PUFAs (polyunsaturated fatty acids) (43). Indeed, 14 of the 25 sequences had hydrophobic ratios above 50%. The highest levels of hydrophobicity were found for the sequences MAPAAVAAAEAGSK (64%), EALEAMFL (75%), LCPVHRAADL (60%), and PAEMVAAALDR (70%). The SH group of Cys (C) has a crucial antioxidant activity due to its direct interaction with radicals (34). The sequence NANGELCPNNMCCSQWGYCGLGSEFCGNGCQSGACCPEK, which was identified in fraction 5 of rye sourdough, contains 8 cysteine residues. It was hypothesized that the antioxidant activity of His-containing peptides is related to the hydrogen-donating, lipid peroxyl radical-trapping, and/or the metal ion-chelating ability of the imidazole group (3, 36). Eight sequences contained His residues. In particular, His at the N terminus was found for the sequences HPVPPKKK and HKEMQAIFDVYIMFIN from fractions 3 of spelt sourdough and 23 of kamut sourdough, and His at the C terminus was found for the sequences KVALMSAGSMH and GVSNAAVVAGGH from fractions 15 of rye sourdough and 2 of kamut sourdough. His at the N terminus acts mainly as a metal ion chelator, while at the C terminus His is an effective scavenger against various radicals (4). Four sequences have Ala or Leu at the N or C terminus, which was already shown to be a typical feature for antioxidant peptides (43, 47). Amino acids with aromatic residues may donate protons to electron-deficient radicals. This property improves the radical-scavenging activity of peptides (43). Sixteen sequences contained one or more aromatic amino acids.
This study shows that selected lactic acid bacteria have the capacity to synthesize antioxidant peptides during the sourdough fermentation of various cereal flours. The fermentation conditions employed here are applicable at the industrial level for making additive-free bakery products with high nutritional value.
The purified peptides exhibited bioactive properties compatible with various antioxidant mechanisms, thus indicating presumptive protection against free radicals. These features could lead to the production of innovative functional foods and the design of new synthetic peptides for food/pharmaceutical applications.
ACKNOWLEDGMENTS
We thank Giammaria Giuliani and Barbara Marzani of Giuliani S.p.A. (Milano, Italy) for support in the ex vivo tests.
Footnotes
Published ahead of print 9 December 2011
REFERENCES
- 1. AACC 2003. Approved methods of the American Association of Cereal Chemistry, 10th ed. AACC, St. Paul, MN [Google Scholar]
- 2. Adebiyi AP, Adebiyi AO, Yamashita J, Ogawa T, Muramoto K. 2009. Purification and characterization of antioxidative peptides derived from rice bran protein hydrolysates. Eur. Food Res. Technol. 228:553–563 [Google Scholar]
- 3. Chan KM, Decker EA. 1994. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 34:403–426 [DOI] [PubMed] [Google Scholar]
- 4. Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. 1998. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 46:49–53 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Know SW, Kim SC, Zhao Y. 2005. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J. Proteome Res. 4:998–1005 [DOI] [PubMed] [Google Scholar]
- 6. Church FC, Swaisgood HE, Porter DH, Catignani GL. 1983. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66:1219–1227 [Google Scholar]
- 7. Coda R, et al. 2008. Long-term fungi inhibitory activity of water-soluble extract from Phaseolus vulgaris cv Pinto and sourdough lactic acid bacteria during bread storage. Appl. Environ. Microbiol. 74:7391–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coda R, et al. 2011. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 77:3484–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. 2005. Physicochemical characterization of casein phosphopeptideamorphous calcium phosphate nanocomplexes. J. Biol. Chem. 280:15362–15369 [DOI] [PubMed] [Google Scholar]
- 10. De Angelis M, et al. 2003. Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 87:259–270 [DOI] [PubMed] [Google Scholar]
- 11. De Angelis M, et al. 2007. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 114:69–82 [DOI] [PubMed] [Google Scholar]
- 12. De Angelis M, et al. 2007. Use of sourdough lactobacilli and oat fibre to decrease the glycemic index of white wheat bread. Br. J. Nutr. 98:1196–1205 [DOI] [PubMed] [Google Scholar]
- 13. De Angelis M, et al. 2006. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue probiotics and gluten intolerance. Biochim. Biophys. Acta 1762:80–93 [DOI] [PubMed] [Google Scholar]
- 14. Di Cagno R, et al. 2004. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 70:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gauthier SF, Pouliot Y, Saint-Sauveur D. 2006. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 16:1315–1323 [Google Scholar]
- 16. Gobbetti M, De Angelis M, Di Cagno R, Rizzello CG. 2008. Sourdough/lactic acid bacteria, p. 267–288 In Arendt EK, Dal Bello F. (ed), Gluten free cereal products and beverages. Academic Press, New York, NY [Google Scholar]
- 17. Gobbetti M, Di Cagno R, De Angelis M. 2010. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 50:716–727 [DOI] [PubMed] [Google Scholar]
- 18. Hansen MB, Nielsen SE, Berg K. 1989. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 119:203–210 [DOI] [PubMed] [Google Scholar]
- 19. Harman D. 2001. Aging: overview. Ann. N. Y. Acad. Sci. 928:1–21 [DOI] [PubMed] [Google Scholar]
- 20. Hettiarachchy NS, Glenn KC, Gnanasambandam R, Johnson MG. 1996. Natural antioxidant extract from fenugreek (Trigonella foenumgraecum) for ground beef patties. J. Food Sci. 61:516–519 [Google Scholar]
- 21. Jia J, et al. 2010. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 119:336–342 [Google Scholar]
- 22. Katina K, et al. 2005. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 16:104–112 [Google Scholar]
- 23. Kits DD, Weiler K. 2003. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 9:1309–1323 [DOI] [PubMed] [Google Scholar]
- 24. Korhonen H, Pihlanto A. 2007. Bioactive peptides from food proteins, p. 5–39 In Hui YH. (ed), Handbook of food products manufacturing. Health, meat, milk, poultry, seafood and vegetables. Wiley Interscience, Hoboken, NJ [Google Scholar]
- 25. McCann KB, et al. 2006. Isolation and characterisation of a novel antibacterial peptide from bovine alphaS1-casein. Int. Dairy J. 16:316–323 [Google Scholar]
- 26. Meisel H, FitzGerald RJ. 2003. Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr. Pharm. Des. 9:1289–1295 [DOI] [PubMed] [Google Scholar]
- 27. Mendis E, Rajapakse N, Kim SK. 2005. Antioxidant properties of a radicals scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 53:581–587 [DOI] [PubMed] [Google Scholar]
- 28. Mikovà K. 2007. The regulation of antioxidants in foods, p 267–283 In Rahman MS. (ed), Handbook of food preservation, 2nd ed. Taylor & Francis Group, Boca Raton, FL [Google Scholar]
- 29. Minervini F, et al. 2003. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolized caseins of milk from six species. Appl. Environ. Microbiol. 69:5297–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitsuta H, Yasumoto K, Iwami K. 1996. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo Shohkuryo 19:210–214 [Google Scholar]
- 31. Osawa T, Namiki M. 1985. Natural antioxidant isolated from eucalyptus leaf waxes. J. Agric. Food Chem. 33:777–780 [Google Scholar]
- 32. Osborne TB. 1907. The proteins of the wheat kernel. Carnegie Institute of Washington, publication 84. Judd and Detweiler, Washington, DC [Google Scholar]
- 33. Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK. 2001. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 78:651–656 [Google Scholar]
- 34. Pasini G, Simonato B, Giannattasio M, Peruffo ADB, Curioni A. 2001. Modifications of wheat flour proteins during in vitro digestion of bread dough, crumb, and crust: an electrophoretic and immunological study. J. Agric. Food Chem. 49:2254–2261 [DOI] [PubMed] [Google Scholar]
- 35. Qian ZJ, Jung WK, Kim SK. 2008. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 6:1690–1698 [DOI] [PubMed] [Google Scholar]
- 36. Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. 2005. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 38:175–182 [Google Scholar]
- 37. Rizzello CG, et al. 2007. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl. Environ. Microbiol. 73:4499–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizzello CG, Cassone A, Di Cagno R, Gobbetti M. 2008. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 16:6936–6943 [DOI] [PubMed] [Google Scholar]
- 39. Rizzello CG, Nionelli L, Coda R, De Angelis M, Gobbetti M. 2010. Effect of sourdough fermentation on stabilisation, chemical and nutritional characteristics of wheat germ. Food Chem. 119:1079–1089 [Google Scholar]
- 40. Rizzello CG, Cassone A, Coda R, Gobbetti M. 2011. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 127:952–959 [DOI] [PubMed] [Google Scholar]
- 41. Rizzello CG, Nionelli L, Coda R, Gobbetti M. 2011. Synthesis of the cancer preventive peptide lunasin by lactic acid bacteria during sourdough fermentation. Nutr. Cancer doi:10.1080/01635581.2012.630159 [DOI] [PubMed] [Google Scholar]
- 42. Salmenkallio-Marttila M, Katina K, Autio K. 2001. Effect of bran fermentation on quality and microstructure of high-fibre wheat bread. Cereal Chem. 78:429–435 [Google Scholar]
- 43. Sarmadi BH, Ismail A. 2010. Antioxidative peptides from food proteins: a review. Peptides 31:1949–1956 [DOI] [PubMed] [Google Scholar]
- 44. Shewry R, Tatham A. 1990. The prolamine storage proteins of cereal seeds: structure and evolution. Biochem. J. 267:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shimada K, Fujikawa K, Yahara K, Nakamura T. 1992. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40:945–948 [Google Scholar]
- 46. Shimizu M, et al. 2009. Antithrombotic papain-hydrolyzed peptides isolated from pork meat. Thromb. Res. 123:753–757 [DOI] [PubMed] [Google Scholar]
- 47. Suetsuna K, Chen JR. 2002. Isolation and characterization of peptides with antioxidant activity derived from wheat gluten. Food Sci. Technol. 8:227–230 [Google Scholar]
- 48. Sun J, He H, Xie BJ. 2004. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 52:6646–6652 [DOI] [PubMed] [Google Scholar]
- 49. Tang CH, Peng J, Zhen DW, Chen Z. 2009. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 115:672–678 [Google Scholar]
- 50. Wanita A, Lorenz K. 1996. Antioxidant potential of 5-N-pentadecylresorcinol. J. Food Process. Preserv. 20:417–429 [Google Scholar]
- 51. Weiss W, Vogelmeier C, Gorg A. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14:805–816 [DOI] [PubMed] [Google Scholar]
- 52. Yu L, et al. 2002. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 50:1619–1624 [DOI] [PubMed] [Google Scholar]
- 53. Zhong F, Liu J, Ma JJ, Shoemaker CF. 2007. Preparation of hypocholesterol peptides from soy protein and their hypocholesterolemic effect in mice. Food Res. Int. 40:661–667 [Google Scholar]