Abstract
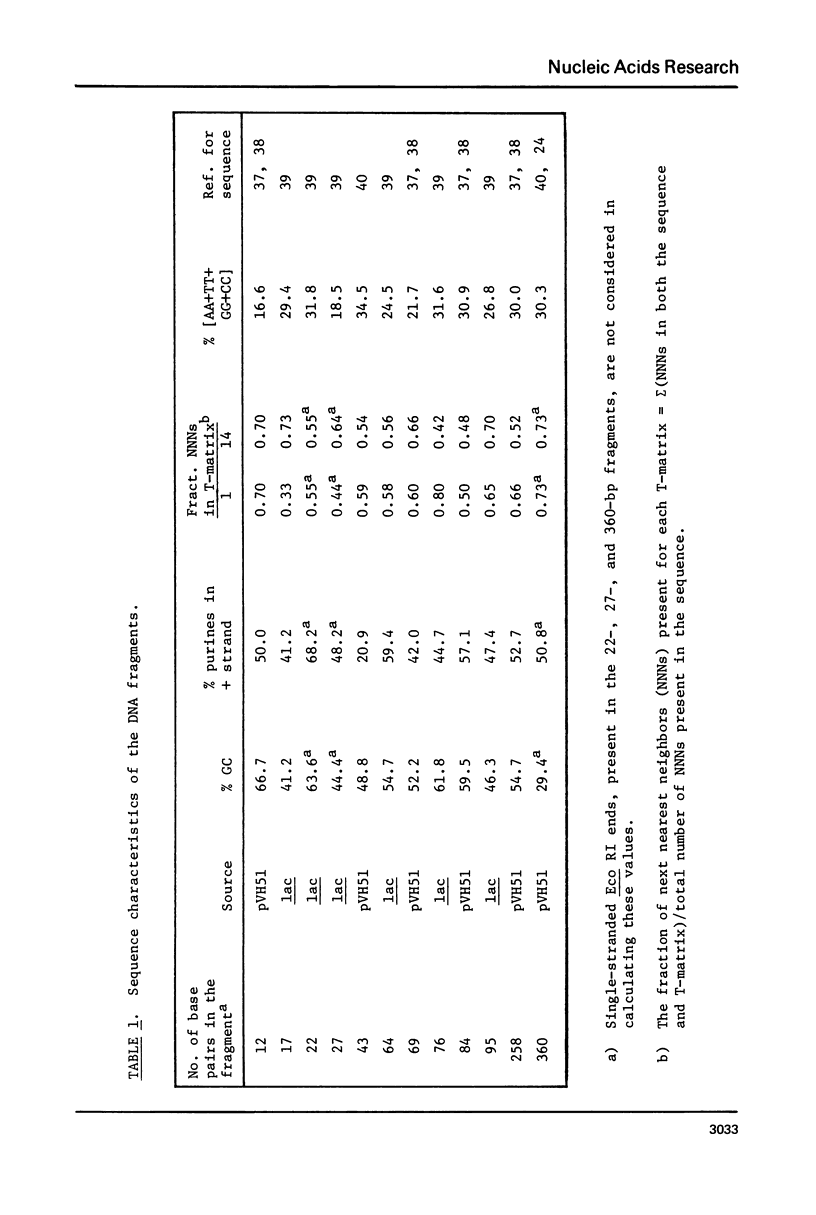
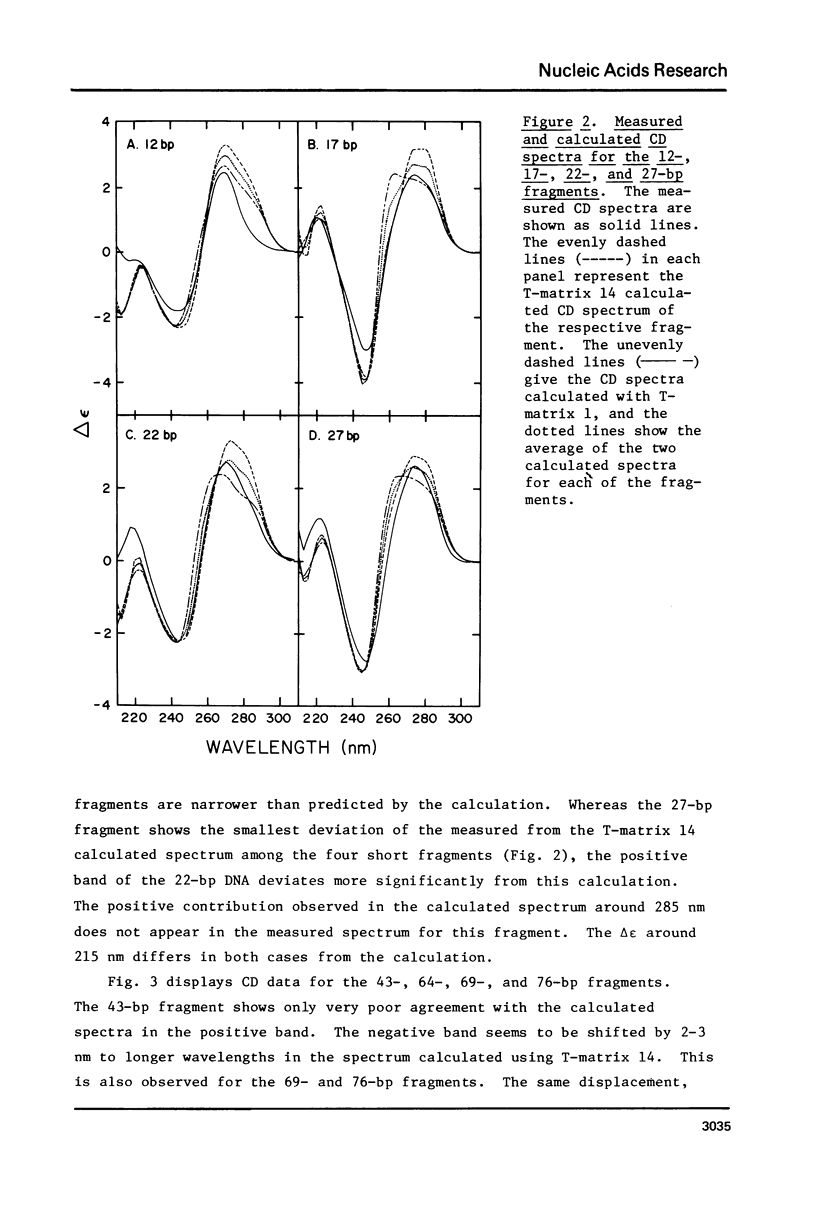
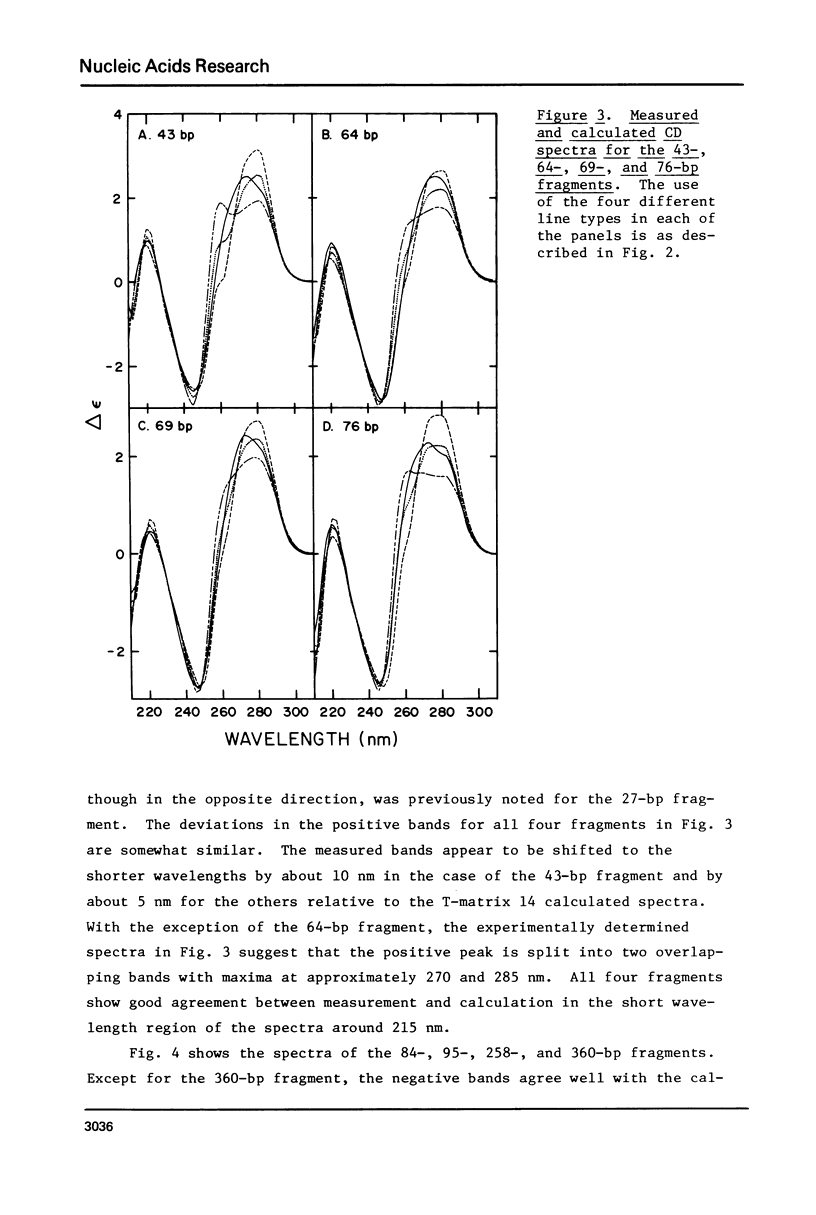
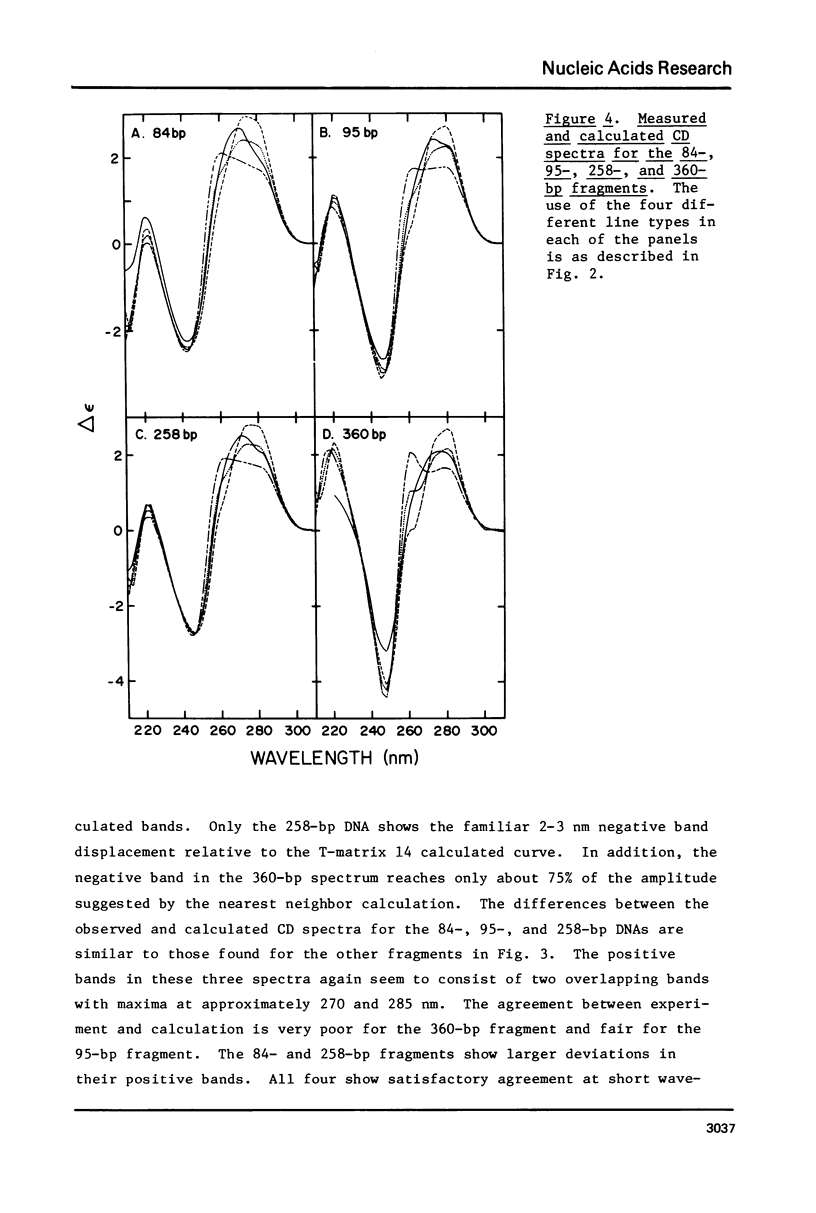
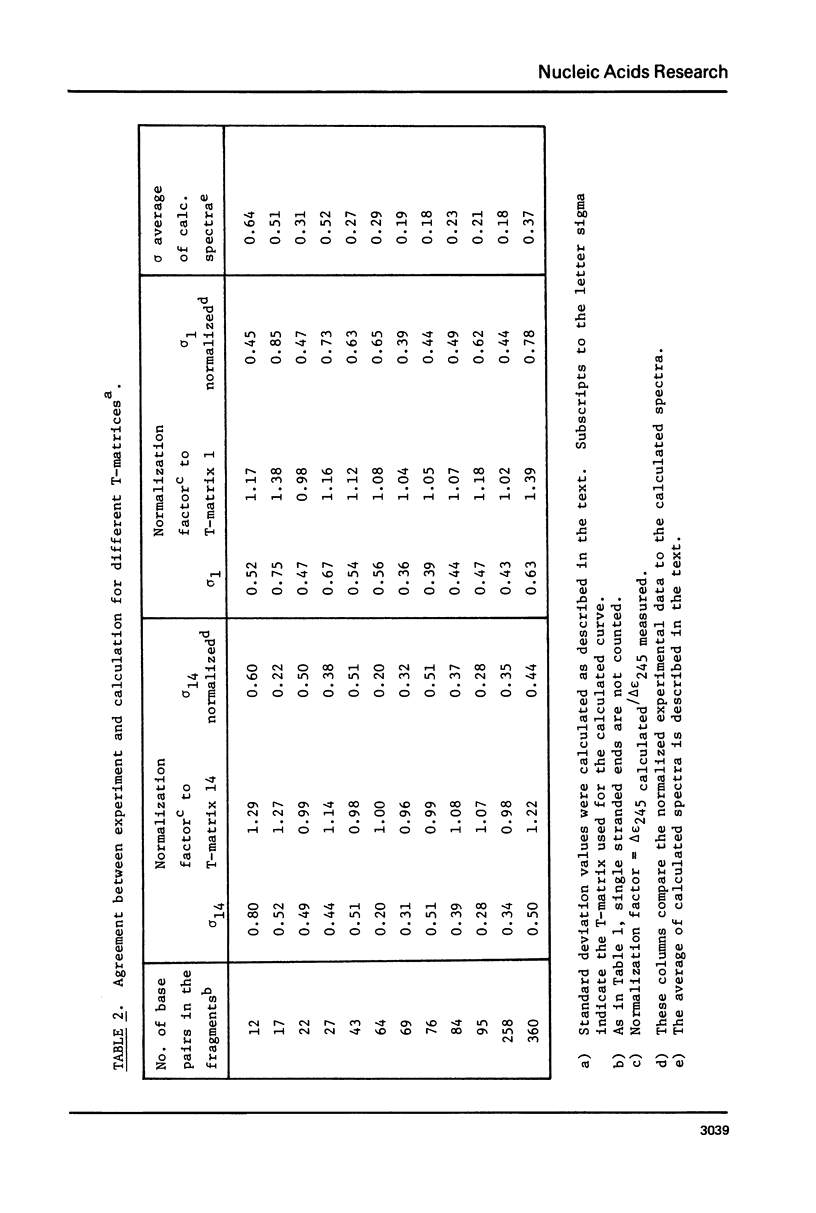
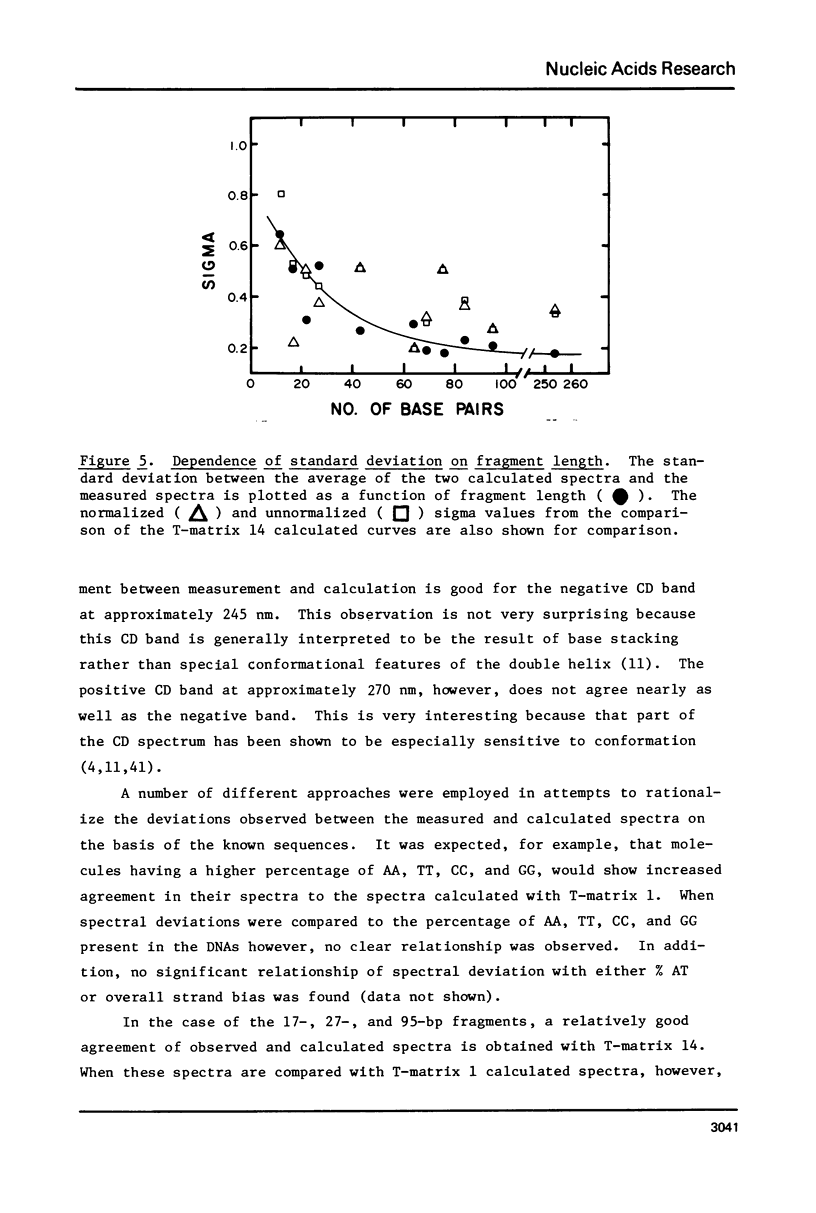
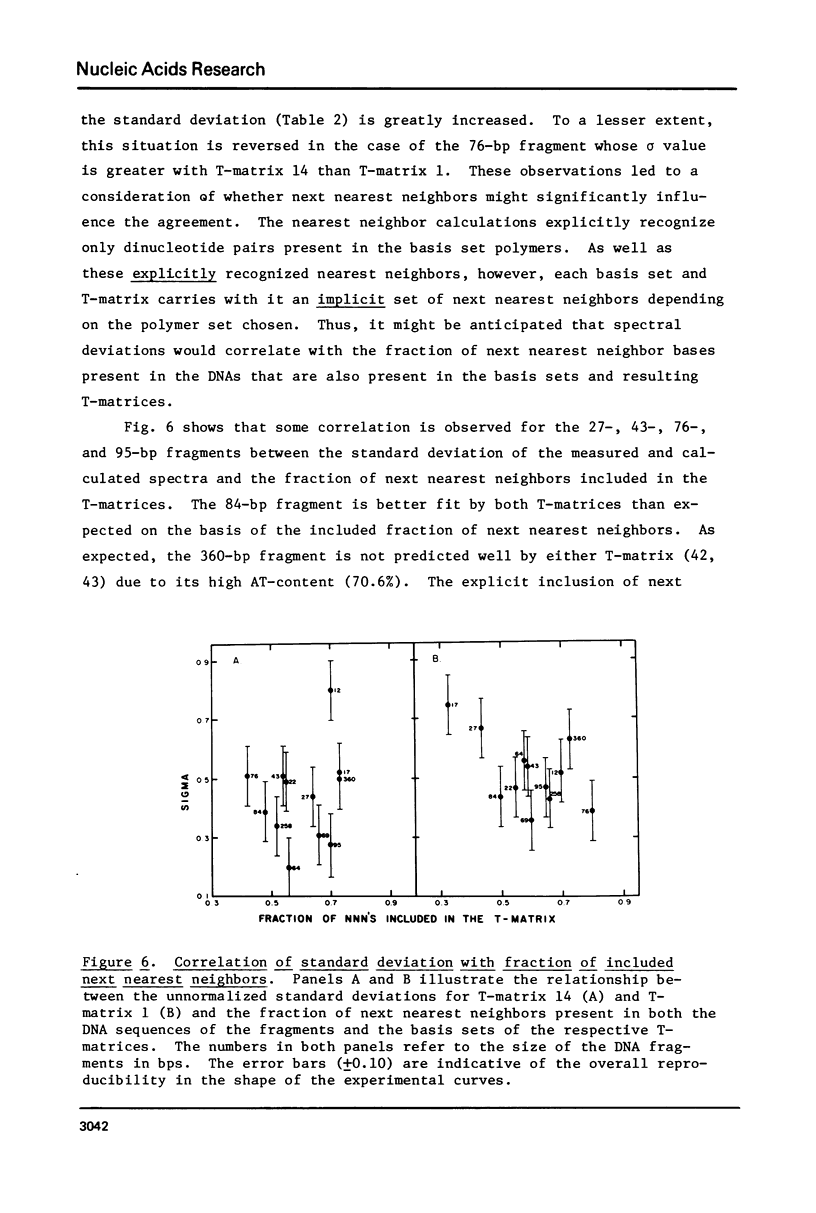
The CD spectra of twelve DNA restriction fragments ranging in size from 12 to 360 base pairs are reported. Since the sequences of these fragments are known, it is possible to calculate their CD spectra from a set of nearest neighbor contributions derived from a combination of synthetic polydeoxyribonucleotides. While the calculations lead to good agreement in the negative band at approximately 245 nm, they generally reproduce the positive band at approximately 270 nm only poorly. The experimentally observed positive band consists of two peaks centered around 270 and 285 nm. The comparison of calculated and measured spectra reveals that end effects lead to increased disagreement for fragments smaller than approximately 40 base pairs. The disagreement between calculated and measured spectra can be partially attributed to the fraction of next nearest neighbors in the DNAs, which are also in the spectral components. Thus, the sequence specific CD contributions in the long wavelength region of the spectra extend at least to next nearest neighbor nucleotides and may extend beyond.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F. S., Gray D. M., Roberts G. P., Tinoco I., Jr The ultraviolet circular dichroism of some natural DNAs and an analysis of the spectra for sequence information. Biopolymers. 1972;11(4):853–879. doi: 10.1002/bip.1972.360110410. [DOI] [PubMed] [Google Scholar]
- Cech C. L., Tinoco I., Jr Circular dichroism calculations for double-stranded polynucleotides of repeating sequence. Biopolymers. 1977 Jan;16(1):43–65. doi: 10.1002/bip.1977.360160105. [DOI] [PubMed] [Google Scholar]
- Chan A., Kilkuskie R., Hanlon S. Correlations between the duplex winding angle and the circular dichroism spectrum of calf thymus DNA. Biochemistry. 1979 Jan 9;18(1):84–91. doi: 10.1021/bi00568a013. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Hillen W., Wells R. D. A 300 MHz and 600 MHz proton NMR study of a 12 base pair restriction fragment: investigation of structure by relaxation measurements. Nucleic Acids Res. 1980 Dec 11;8(23):5795–5812. doi: 10.1093/nar/8.23.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Hamilton F. D., Vaughan M. R. The analysis of circular dichroism spectra of natural DNAs using spectral components from synthetic DNAs. Biopolymers. 1978 Jan;17(1):85–106. doi: 10.1002/bip.1978.360170107. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Morgan A. R., Ratliff R. L. A comparison of the circular dichroism spectra of synthetic DNA sequences of the homopurine . homopyrimidine and mixed purine- pyrimidine types. Nucleic Acids Res. 1978 Oct;5(10):3679–3695. doi: 10.1093/nar/5.10.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Hillen W., Goodman T. C., Wells R. D. High resolution thermal denaturation analyses of small sequenced DNA restriction fragments containing Escherichia coli lactose genetic control loci. J Biol Chem. 1979 Oct 25;254(20):10128–10134. [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Hardies S. C., Wells R. D. Preparation and characterization of large amounts of restriction fragments containing the E. coli lac control elements. Gene. 1979 Sep;7(1):1–14. doi: 10.1016/0378-1119(79)90039-8. [DOI] [PubMed] [Google Scholar]
- Helleiner C. W., Verpoorte J. A. Circular dichroism spectra of DNA of high adenine plus thymine content. Can J Biochem. 1974 Jul;52(7):582–585. doi: 10.1139/o74-084. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Chow L., Helinski D. R. Characterization of a mini-ColC1 plasmid. J Bacteriol. 1976 Apr;126(1):447–453. doi: 10.1128/jb.126.1.447-453.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Goodman T. C., Benight A. S., Wartell R. M., Wells R. D. High resolution experimental and theoretical thermal denaturation studies on small overlapping restriction fragments containing the Escherichia coli lactose genetic control region. J Biol Chem. 1981 Mar 25;256(6):2761–2766. [PubMed] [Google Scholar]
- Hillen W., Goodman T. C., Wells R. D. Salt dependence and thermodynamic interpretation of the thermal denaturation of small DNA restriction fragments. Nucleic Acids Res. 1981 Jan 24;9(2):415–436. doi: 10.1093/nar/9.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Wells R. D. Circular dichroism studies of the B goes to A conformational transition in seven small DNA restriction fragments containing the Escherichia coli lactose control region. Nucleic Acids Res. 1980 Nov 25;8(22):5427–5444. doi: 10.1093/nar/8.22.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G. T., Wells R. D. The leftward promoter of bacteriophage lambda. Isolation on a small restriction fragment and deletion of adjacent regions. J Biol Chem. 1981 Feb 25;256(4):1998–2002. [PubMed] [Google Scholar]
- Horn G. T., Wells R. D. The leftward promoter of bacteriophage lambda. Structure, biological activity, and influence by adjacent regions. J Biol Chem. 1981 Feb 25;256(4):2003–2009. [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. Nucleotide sequence of the region required for maintenance of colicin E1 plasmid. Mol Gen Genet. 1979 Oct 3;176(2):161–170. doi: 10.1007/BF00273210. [DOI] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cloning and localization of the in vitro functional origin of replication of bacteriophage T7 DNA. J Biol Chem. 1979 Jun 25;254(12):5555–5561. [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Identification, cloning and characterization of three late promoters at 14.6, 14.8 and 15.9% of T7 DNA. J Mol Biol. 1979 Nov 25;135(1):91–109. doi: 10.1016/0022-2836(79)90342-5. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Recognition and initiation site for four late promoters of phage T7 is a 22-base pair DNA sequence. Nature. 1979 Jul 5;280(5717):35–39. doi: 10.1038/280035a0. [DOI] [PubMed] [Google Scholar]
- Patient R. K. Characterization of in vitro transcription initiation and termination sites in Col E1 DNA. Nucleic Acids Res. 1979 Jun 25;6(8):2647–2665. doi: 10.1093/nar/6.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient R. K., Hardies S. C., Larson J. E., Inman R. B., Maquat L. E., Wells R. D. Influence of A-T content on the fractionation of DNA restriction fragments by RPC-5 column chromatography. J Biol Chem. 1979 Jun 25;254(12):5548–5554. [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Sprecher C. A., Baase W. A., Johnson W. C., Jr Conformation and circular dichroism of DNA. Biopolymers. 1979 Apr;18(4):1009–1019. doi: 10.1002/bip.1979.360180418. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Hardies S. C., Horn G. T., Klein B., Larson J. E., Neuendorf S. K., Panayotatos N., Patient R. K., Selsing E. RPC-5 column chromatography for the isolation of DNA fragments. Methods Enzymol. 1980;65(1):327–347. doi: 10.1016/s0076-6879(80)65043-5. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. VI. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim Biophys Acta. 1974 Aug 15;361(1):11–32. [PubMed] [Google Scholar]



