Figure 4.
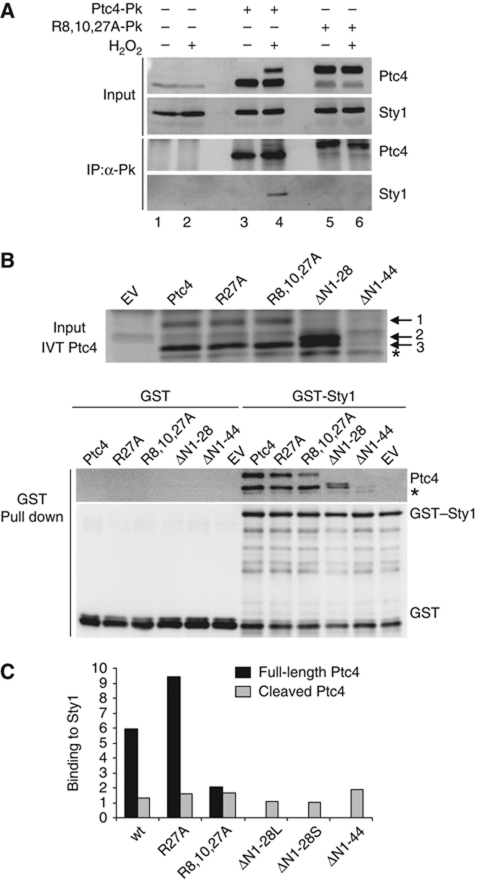
Sty1 preferentially binds to the full-length form of Ptc4. (A) Sty1 and Ptc4 interact in vivo. Ptc4 was immunoprecipitated using anti-V5 agarose from protein extracts prepared from the following strains: wild type, ptc4-3Pk or ptc4R8A,R10A,R27A-3Pk (R8,10,27A-Pk). Cells were stressed by the addition of H2O2 for 30 min as indicated. Whole cell extracts (input) and immunoprecipitated proteins (IP:α-Pk) were analysed by immunoblotting. Ptc4 and Sty1 were detected with anti-Pk and anti-Hog1 antiserum, respectively. Results are representative of three independent experiments. (B) Ptc4 and Sty1 interact in vitro. Top panel: in-vitro translation of Ptc4-3Pk in reticulocyte lysates. Wild-type or various mutant forms were synthesized and examined by western blotting. Ptc4 was detected using anti-Pk antiserum. EV represents in-vitro translation reactions using an empty vector. The asterisk indicates a Ptc4 product that is generated from an internal start site, presumably at methionine 45. Band 1 is the full-length form of Ptc4; band 3 is a processed form that lacks the MTS and probably arises from cleavage by proteases present in the reticulocyte lysate; we do not know what form of Ptc4 band 2 represents. Lower panel: GST or GST–Sty1 was expressed in E. coli. The purified proteins were mixed with in-vitro translated Ptc4. The GST proteins were then precipitated on glutathione sepharose and the amount of Ptc4 bound to Sty1 was assessed by western blotting. (C) Ptc4 binding to Sty1 was calculated from (B) by comparing the intensity of the signal of Ptc4 bound to Sty1 with the intensity of the Ptc4 input band. The amount of binding was corrected for the amount of GST–Sty1 precipitated in each case. The results are representative of three independent experiments. ΔN1-28L and ΔN1-28S refer to the upper and lower bands, respectively. The black and grey bars represent the Sty1-binding ability of the full-length and cleaved isoforms of Ptc4, respectively. Figure source data can be found in Supplementary data.