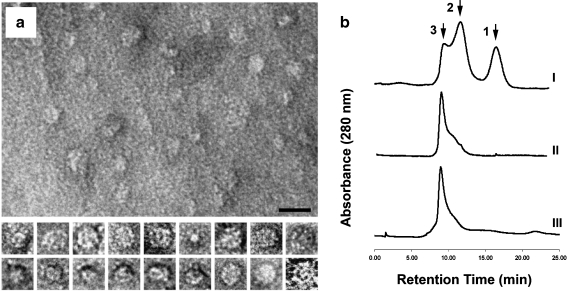
Fig. 3.
Characterization of the protein oligomers spontaneously formed by IbpA subunits. a Electron micrographs of the oligomers, negative staining (1% aqueous uranyl acetate). At the upper part, a panoramic picture with the oligomers is displayed. The bar corresponds to 20 nm. At the lower part, representative examples of selected individual oligomers (approximately 15 nm in diameter each) exposed at different angles to the plane of a grid. b Evaluation of the oligomers molecular mass by gel filtration (1 × PBS, 1 ml/min). I Chromatographic pattern 10 min after purification of the recombinant IbpA from E. coli cell extract, II 24 h after purification (storage at +4°C), III the same prep under heat treatment (43°C). Peak 1 corresponds to a fraction of monomers or dimers (20–40 kDa), peak 2 represents some intermediate complexes that are formed during the oligomerization process (approx. 250 kDa), and peak 3, obviously, corresponds to a mature oligomeric form of IbpA (more than 400 kDa)