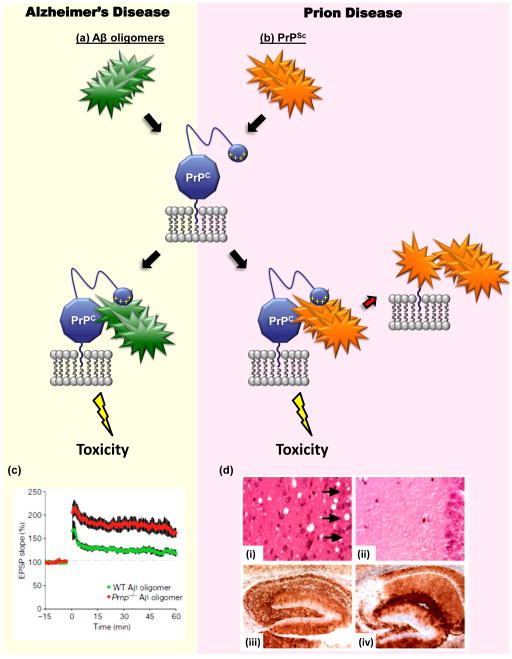
FIGURE 4. PrPC may mediate the toxicity of both Aβ oligomers and PrPSc.
The diagram illustrates how cell-surface PrPC, as a consequence of its binding to either Aβ oligomers (green) or PrPSc (orange), may deliver a neurotoxic signal in Alzheimer’s disease (a) and prion diseases (b), respectively. The N-terminal polybasic domain of PrPC (depicted as a small blue ball carrying positive charges) has been shown to participate in binding to both PrPSc [128] and Aβ oligomers [94]. In addition to acting as a transducer of PrPSc-induced neurotoxicty, PrPC also serves as a precursor for the generation of more molecules of PrPSc, which allows prion propagation [1] [(red arrow in part (b)]. (c) PrPC is required for inhibition of hippocampal LTP induced by Aβ oligomers. Field potentials were recorded from the CA1 region of hippocampal slices derived from wild-type mice (green) or Prn-p−/− mice (red), which had both been treated with Aβ oligomers [4]. The red trace is similar to the one recorded from untreated control (Prn-p+/+) slices (not shown). (d) Depletion of neuronal PrPC reverses spongiosis, but not accumulation of PrPSc, in prion-infected mice [7]. Neuronal PrPC expression in mice previously infected with scrapie prions was eliminated by means of Cre-Lox recombination at approximately 12 weeks of age. Hippocampal sections from PrP-expressing mice (i and iii) or PrP-ablated mice (ii and iv) (at 8 and 48 weeks post infection, respectively) were stained with hematoxylin and eosin (i and ii) or an anti-PrP antibody (iii and iv). The severe loss of CA1 to CA3 neurons caused by scrapie infection (arrows) can be rescued by depleting neuronal PrPC, despite continued PrPSc accumulation. Reproduced, with permission, from [4] (c) and [7] (d).