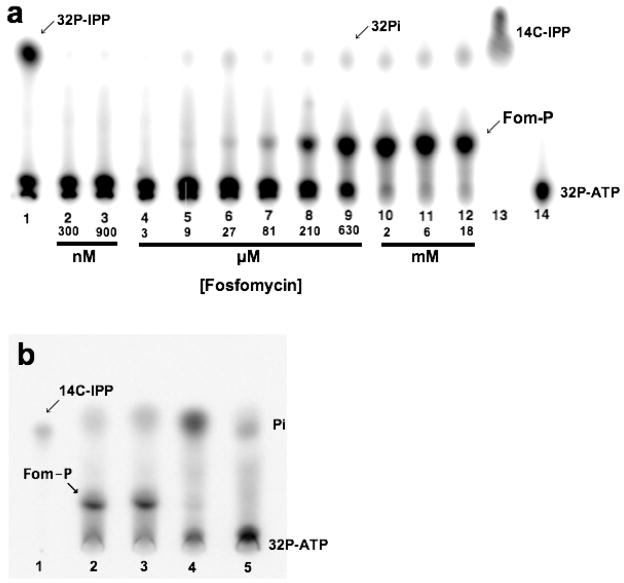
Figure 2.
Autoradiograms showing that IPK can phosphorylate fosfomycin in the presence of ATP. (a) Product turnover assay of THA IPK using IP or fosfomycin as substrates. The product of the native reaction, [32P] IPP, and the by-product of the intrinsic ATPase activity, 32Pi, migrate with almost similar Rfs. This ATPase activity is independent of fosfomycin concentration. Increasing phosphorylation of fosfomycin is observed in the μM to mM range. All lanes except lane 13 contained γ-[32P] ATP and 200 nM IPK. (1) 3.5 μM IP and IPK, (2) 300 nM fosfomycin, (3) 900 nM, (4) 3 μM, (5) 9 μM, (6) 27 μM, (7) 81 μM, (8) 210 μM, (9) 630 μM, (10) 2 mM, (11) 6 mM, (12) 18 mM, (13) [14C-IPP] (14) γ-[32P] ATP and IPK. (b) Comparison of the products of S. wedmorensis FomA and THA IPK (20 μM each) upon incubation with fosfomycin. All lanes except lane 1 contained γ-[32P] ATP. (1) [14C] IPP standard. (2) S. wedmorensis FomA and 10 mM fosfomycin, (2) THA IPK and 10 mM fosfomycin, (3) S. wedmorensis FomA incubated without fosfomycin, and (4) incubation including 10 mM fosfomycin but with neither S. wedmorensis FomA or THA IPK.