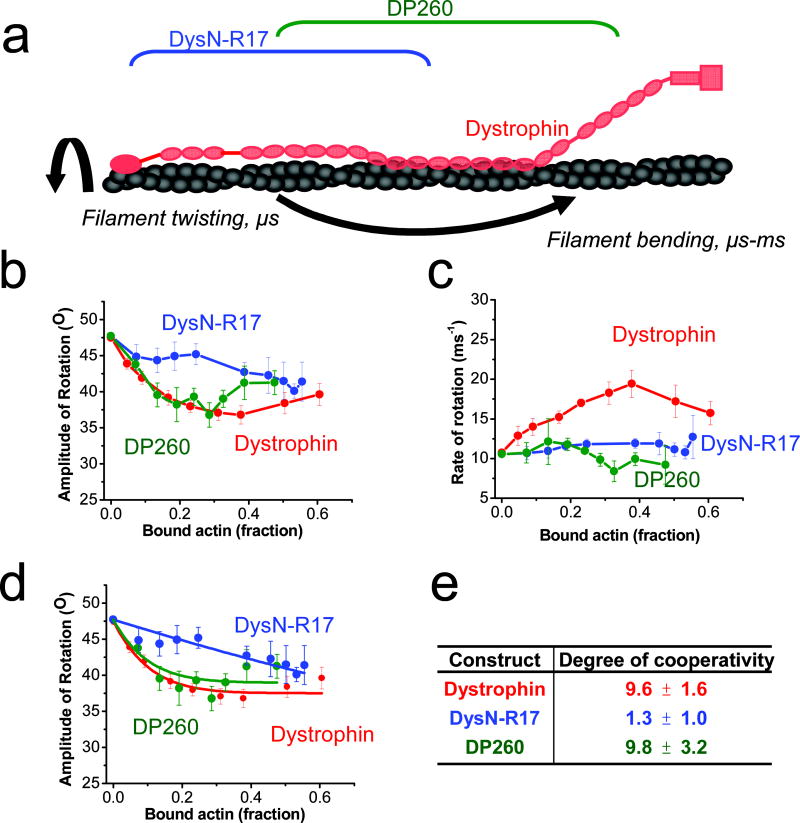
Fig. 5. TPA shows that the C-terminal region of dystrophin contributes to cooperative restriction of actin rotational amplitude.
(a) Diagram of actin rotational dynamics evaluated by time-resolved phosphorescence anisotropy (TPA) when bound to full length dystrophin (red), DysN-R17 (blue) or DP260 (green). Effects on amplitudes (b) and rates (c) of actin rotational motion are plotted against the fraction of bound actin protomers (Eq 5). Full length dystrophin (red) restricts the amplitude and increases the rate of actin rotational motion 16. Deletion of the C-terminal region of dystrophin in DysN-R17 (blue) shows similar restriction of amplitude (b) at higher titrations compared with full length dystrophin, but there is loss in the cooperativity of the effect. DP260 (green), containing the C-terminal tail regains the cooperative effect on restricting actin rotational amplitude. However, either loss of the C-terminal or N-terminal regions in DysN-R17 or DP260 fails to produce any increase on the rate of rotational motion in actin (c). The degree of cooperativity (n) was determined by fitting to the equilibrium binding constant (Eq. 5) (d) and summarized in (e). The results from the fits in (d) show that DysN-R17 loses considerable cooperativity in its effect on rotational amplitude of actin filaments compared with dystrophin. On the other hand, DP260 with the C-terminal region intact restores the degree of cooperativity seen in full length dystrophin in restricting the angular amplitude of rotational motion.