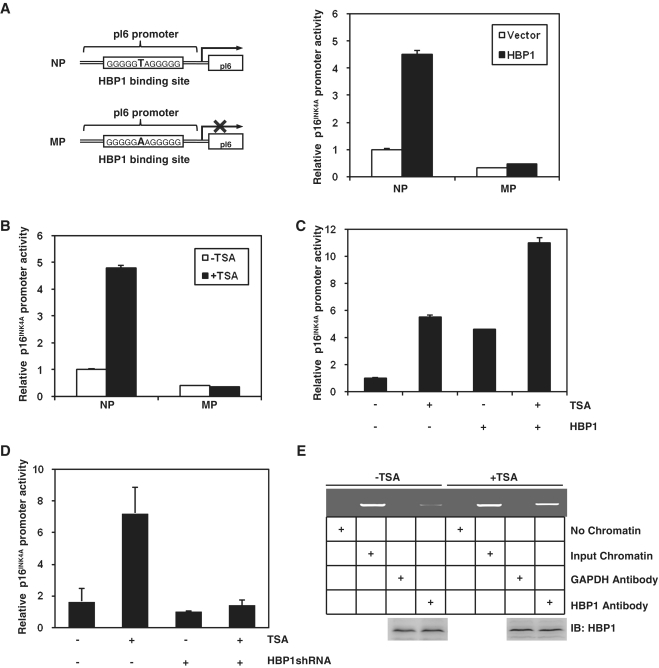
Figure 2.
TSA increased HBP1 transactivation on p16INK4A by enhancing the DNA binding of HBP1 to the promoter region of p16INK4A. (A) The integrity of binding site is indispensible for HBP1 enhancing p16INK4A promoter. Schematic diagrams of the native p16INK4A promoter (NP) and its mutant promoter (MP) (left panel). The mutant promoter was constructed by changing one point (T to A) in HBP1-binding site in the background of native p16INK4A promoter. Luciferase assay was performed in cells transfected with NP, or MP and HBP1 to test p16INK4A mutant promoter (right panel). (B) HBP1-binding site is required for activating TSA-induced p16INK4A expression. Thirty hours after transfection with either pGL3-NP or pGL3-MP, 2BS cells were treated with TSA at 1 μM for 18 h. Relative luciferase activity was measured after TSA treatment. (C) The transactivation of HBP1 on p16INK4A is enhanced by TSA. Thirty hours after transfection with HBP1, cells were treated with TSA at 1 μM for 18 h. Then the cells were harvested for luciferase assay. (D) TSA loses its transactivation ability at the p16INK4A promoter when HBP1 was knocked-down with shRNA. Thirty hours after transfection with HBP1shRNA, cells were treated with TSA at 1 μM for 18 h. Then the cells were harvested for luciferase assay. (E) TSA enhances the DNA binding of HBP1 to the promoter region of p16INK4A in vivo. ChIP assay with antibody against HBP1 was performed in 2BS cells with or without TSA treatment at 1 μM for 18 h. Lanes with input chromatin were positive controls (lanes 2, 6). Lanes without chromatin or GAPDH antibody were used for negative controls (lanes 1, 3, 5, 7). At the bottom, anti-HBP1 western immunoblotting (IB) for HBP1 protein expression is shown.