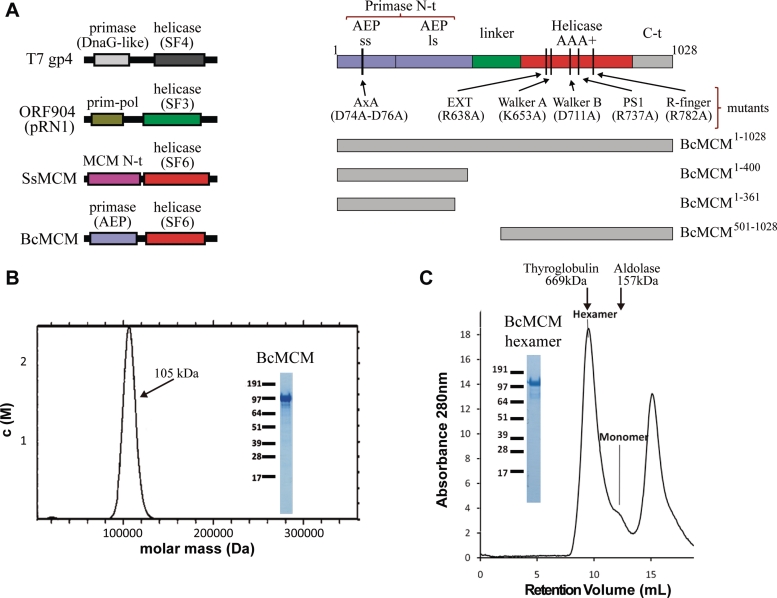
Figure 1.
BcMCM is purified as a monomer but can hexamerize. (A) Graphical representations of the domain architecture present in several multimodular helicases (left panel). The families of associated primases and helicases are indicated. BcMCM presents an exclusive combination of an archaeo–eukaryotic primase (AEP), including the small (ss) and large (ls) subunits, and a MCM-like helicase (Super Family 6). Scheme of the domain arrangement of BcMCM (right panel). A representation of the different constructions and mutants used along this article is depicted in the right lower panel. (B) Analytical ultracentrifugation experiments shows that the recombinant protein BcMCM expressed in E. coli is a monomer. Only one species is detected in the calculated molar mass distribution, c(M), which is consistent with the molecular mass of a monomer (see ‘Materials and Methods’ section). (C) Size-exclusion chromatography illustrates conversion of BcMCM monomers into hexamers. Upon addition of ATPγS and ssDNA (dT)40 and incubation with the purified monomer fractions the BcMCM protein elutes mainly as a hexamer from Superdex 200 column. The hexamer fractions were collected and analysed using SDS–PAGE (inset). The peak at the end of the chromatogram corresponds to the excess of ssDNA used in the assay. Elution positions of the molecular weight standards Thyroglobulin and Aldolase are indicated in the graph.