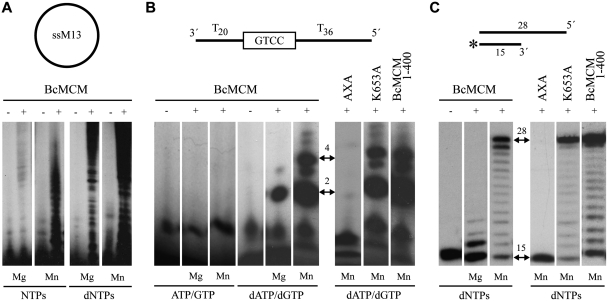
Figure 4.
BcMCM has intrinsic primase and polymerase activity. (A) Circular single-stranded M13 was used as template for BcMCM primase activity. BcMCM primase uses dNTPs more efficiently than NTPs and the reaction is favoured in the presence of manganese as cofactor. (B) A 60-mer ssDNA oligonucleotide (see scheme) was used as template with a putative priming site at the boxed sequence. The wild-type BcMCM displayed an intrinsic primase that preferentially uses dNTPs as substrates and manganese as metal activator. The primase activity is eliminated by the double mutation AxA in the catalytic site. In the monomeric Walker A mutant (K653A) and in the truncated version BcMCM1–400 the primase activity was similar. Arrows indicate the main formation of a dinucleotide and a 4-mer products. (C) Using a conventional template/primer substrate (see scheme), BcMCM displayed an intrinsic DNA polymerization activity, which was absent in the mutant AxA. The polymerase activity is preferentially activated by manganese ions. The DNA synthesis of the hexameric BcMCM is similar to the monomeric Walker A mutant (K653A), thus DNA polymerase activity does not involve hexamerization. The DNA polymerase activity of BcMCM, as in the case of the primase activity, is normal in the truncated version BcMCM1–400. Therefore both the primase and polymerase activities do not require the helicase domain. The initial substrate and the complete product positions are depicted with two-headed arrows. The asterisk in the substrate sketch indicates the labelled primer.