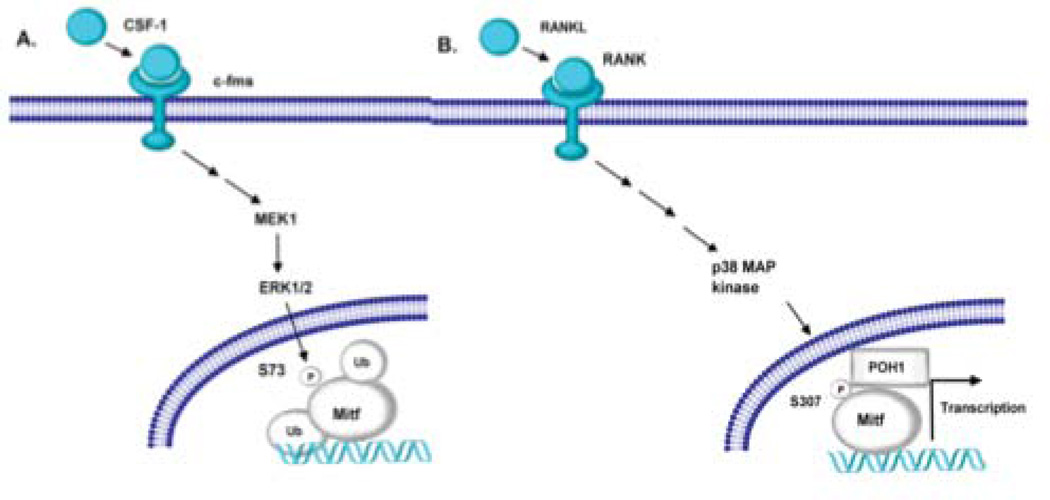
Figure 7. Model of Mitf and POH1 interaction.
(A) M-CSF stimulation of osteoclast activates ERK1/2, which phosphorylates Mitf on serine residue 73 [Weilbaecher et al., 2001]. The serine at residue 73 has been shown to be necessary for Mitf to be ubiquinated [Xu et al., 2000]. (B) RANKL stimulation activates the p38MAPK pathway resulting in Mitf phosphorylated on serine residue 307 [Mansky et al., 2002]. We hypothesize that POH1 interacts with Mitf when it is phosphorylated on serine residue 307. Mitf’s interaction with POH1 prevents Mitf’s ubiquitination allowing for persistent activation of genes necessary for osteoclast differentiation.