Abstract
A hallmark of the adaptive immune response is rapid and robust activation upon rechallenge. In the current issue of Immunity van der Windt et al. (2012) provide an important link between mitochondrial respiratory capacity and the development of CD8+ T cell memory.
At one time, a right-of-passage for successfully mastering basic biochemistry was memorizing (at least long enough to recite on an exam) the Tricarboxylic acid (TCA) cycle and associated pathways leading to the generation of cellular ATP. The current work of the Pearce lab is forcing us to dust off our old Stryer and/or Lehninger tomes (or is there an App. for that?) in order to reexamine the role of these pathways in the development of T cell memory.
Perhaps only rivaled by cancer cells, lymphocyte activation requires an extraordinary amount of energy and biochemical substrates to facilitate expansive cellular division (Fox et al., 2005). Similar to cancer cells, T cells employ aerobic glycolysis as a means of not only generating ATP but also as a mechanism for providing substrates for the generation of nucleic acids, fats and proteins. Indeed, an integral aspect of CD28-mediated costimulation is not only the elaboration of cytokines but also the upregulation of glucose transporters and the activation of biochemical pathways necessary to support these metabolic demands (Frauwirth and Thompson, 2004). Along these lines, more recently, a critical role for myc in the upregulation of metabolic machinery necessary for T cell activation has been described (Wang et al., 2011). It was shown that myc-mediated transcription plays a critical role in the upregulation of genes responsible for driving glycolysis but is not essential for Fatty Acid Oxidation (FAO) and increasing the Oxygen Consumption Rate (OCR). Likewise, mammalian target of rapamycin (mTOR) activation which has been shown to play an important role in regulating CD4+ T effector cell generation also plays an important role in the expression of proteins involved in glycolysis and glucose uptake (Powell et al., 2011). Thus, it is clear that increases in the metabolic machinery are not simply the consequences of T cell activation but actually play an integral role in promoting T cell activation (Fox et al., 2005). Along these lines it has been shown that in addition to failing to produce cytokines upon rechallenge, anergic T cells fail to express the metabolic machinery necessary for T cell activation (Zheng et al., 2009). In other words, the upregulation of metabolic programs promotes the activation of T cells while the inhibition of such programs inhibits T cell function.
The initial antigen encounter leads to a massive increase in the frequency of CD8+ effector cells (Araki et al., 2010). Following this expansion there is a contraction phase which ultimately results in the emergence of long living CD8+ memory T cells with the capability to respond rapidly and robustly upon secondary rechallenge. Thus, memory cells have a unique set of metabolic demands. On the one hand they must employ pathways that facilitate their long-term survival. On the other hand they must respond upon rechallenge even more vigorously than naïve T cells. Van der Windt et al. sought to determine the role of metabolism in regulating the generation and maintenance of memory cells. Their studies reveal that memory CD8+ T cells possessed a markedly increased mitochondrial Spare Respiratory Capacity (SRC) when compared to effector T cells. SRC refers to the extra mitochondrial ability in a cell to generate energy under conditions of great demand. That is, SRC can be thought of as measuring how close a cell is to its “bioenergetic limit” (Nicholls, 2009).
The increase in SRC is dependent upon interleukin-15 (IL-15) signaling which is already known to play a critical role in the generation of CD8+ T cell memory. The generation of memory CD8+ T cells by exposure to IL-15 concomitantly led to an increase in mitochondrial biogenesis. When compared to CD8+ effector cells, memory cells were shown to have increased mitochondrial membrane potential and less superoxide production which in turn contributed to their increased long term survival. Likewise, while effector cells employed aerobic glycolysis, memory cells switched to oxidative phosphorylation as a means of generating energy. Interestingly, upon rechallenge, the memory cells reverted quickly back to aerobic glycolysis. Furthermore, IL-5-induced memory cells were better prepared for this marked increase in energetic demand in that they had increased ATP upon re-stimulation when compared to (IL-2 promoted) T effector cells. Thus the increased SRC in memory cells leads to increased ATP upon rechallenge providing energy for rapid and robust secondary activation.
Previously this group had shown that Fatty Acid Oxidation (FAO) promotes memory cell generation (Pearce et al., 2009). Specifically, it was shown that activated CD8+ T cells lacking the TNF receptor TRAF6 fail to develop into memory cells. This defect was linked to defective AMP-activated kinase activation and subsequently mitochondrial FAO. As such, in the current work, it was hypothesized that IL-15-induced SRC might be associated with an increase in mitochondrial FAO. Indeed, inhibiting FAO with the drug etomoxir led to a decrease in SRC in memory cells in both the activated and resting state. Such findings demonstrate the necessity for FAO in generating energy in memory cells. In as much as IL-15 promoted the increase in mitochondrial biogenesis, the ability of IL-15 to regulate FAO was examined. The rate limiting step in the transfer of fatty acids from the cytosol into the mitochondria is mediated by carnitine palmitoyltransferase 1 (CPT1a). Indeed, it was found that IL-15 signaling led to the upregulation of CPT1a expression. Functionally, silencing CPT1a led to decreases in the OCR and SRC of memory cells while overexpression of CPT1a led to increases in OCR and SRC in both memory and IL-2 induced effector cells. Likewise, the overexpression of CPT1a led to an increase in survival of memory cells. Specifically, in an in vivo model of infection, overexpression of CPT1a led to a mitigated contraction phase and thus an overall long term increase in memory cells. Thus, IL-15-induced CPT1a is revealed as playing a critical role in the development of CD8+ T cell memory. Interestingly, and not entirely unexpectedly, the ability of IL-15 to promote memory cell differentiation wasnot exclusively related to CPT1a expression. When CPT1a overexpressing cells were transferred into IL-15 deficient mice, they did not survive to the same extent as bona fide memory cells. Thus the full extent of the IL-15-induced signaling program that promotes the generation of memory cells remains to be determined. Likewise the precise connections linking FAO with T cell differentiation as well as the role of the availability of various carbon sources on guiding T cell differentiation and function still need to be elucidated.
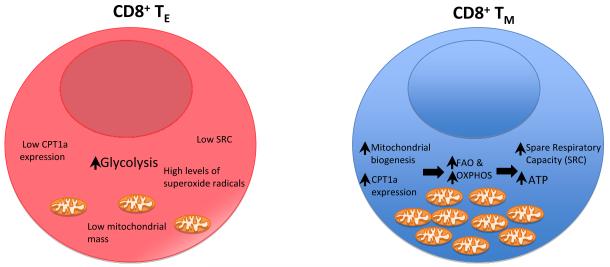
Overall, this work provides important insight in terms of understanding the role of metabolism in regulating immunologic function (Figure 1). Activated effector cells are anabolic, employing primarily glucose as their carbon source and glycolysis as a means of generating ATP (Fox et al., 2005). Memory cells are catabolic, able to metabolize fatty and amino acids in addition to glucose and employ Oxidative Phosphorylation (the TCA cycle) to generate ATP. IL-15 promotes the generation of memory cells by promoting mitochondrial biogenesis and the expression of CPT1a the rate limiting enzyme in FAO. Consequently, memory cells generate less toxic superoxide radicals, live longer and generate increased concentrations of ATP upon secondary rechallenge. This study along with others identifying the role of mTOR, Estrogen Related Receptor-Alpha and myc in regulating lymphocyte metabolism suggest that targeting metabolic pathways may prove to be an effective means of pharmacologically redirecting immunologic responses (Michalek et al., 2011; Powell et al., 2011; Wang et al., 2011). By inhibiting specific metabolic pathways one might on the one hand block undesirable responses, for example in the case of autoimmunity and on the other hand enhance immunity, for example in the case of generating memory cells after vaccination. Furthermore, the classification of different cell types based on metabolic criteria may diverge from phenotypic classifications based strictly on immunologic criteria. As a little “fuel for thought”, based on metabolic requirements it may turn out that Regulatory T cells and CD8+ memory T cells are much more similar then might have been expected.
Figure. Metabolic view of CD8+ effector versus memory cells.
From an immunologic perspective, upon activation CD8+ effector cells are short lived killers able to rapidly proliferate and secrete cytokines. Memory cells on the other hand are long lived cells circulating throughout the lymphoid tissue. Upon activation however, they too rapidly proliferate, secrete cytokines and kill targets. The work of van der Windt et al. paints a contrasting metabolic portrait of these two cell types. IL-2-promoted effector cells employ primarily glucose for fuel and aerobic glycolysis for energy and raw materials. IL-15 promoted memory cells demonstrate increased mitochondrial mass, employ oxidative phosphorylation and fatty acid oxidation with increased expression of of CPT1a. Overall this results in an increase in spare respiratory capacity, an increase in ATP and a decrease in superoxide generation. The net result is that memory cells are built for long term survival and poised for secondary rechallenge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energymetabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. JImmunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, Dwyer MA, Nelson ER, Pollizzi KN, Ilkayeva O, et al. Estrogen-relatedreceptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci U S A. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acidmetabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation ofImmune Responses by mTOR. Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The Transcription FactorMyc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Immunity this issue. 2012 doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic Tcells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]