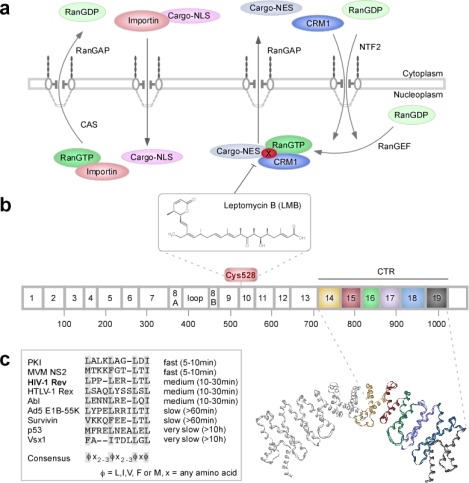
Figure 1.
(a) Schematic illustration of the regulation of nucleo-cytoplasmic transport. As an example for nuclear export, the CRM1-dependent pathway is depicted. (b) Heat-repeat map of CRM1, which is predicted to contain 19 repeats separated by a loop within repeat 8. (c) Kinetic classification of NES sequences established by microinjection experiments of recombinant GST-NES-GFP substrates into the nucleus. Amino acids essential for function are highlighted in grey, putative consensus sequence for leucine-rich NES is shown below.
(a) NLS-bearing cargoes are imported via binding to importins. In the nucleus, the importin-cargo complex dissociates upon RanGTP binding. CAS exports the respective importin back into the cytoplasm. RanGAP hydrolyses RanGTP to RanGDP resulting in release of the importin from CAS. Leucine-rich NES-bearing cargoes bind to CRM1 in the presence of RanGTP, and the complex translocates into the cytoplasm, where the cargo is released following hydrolysis of RanGTP to RanGDP. RanGDP is reimported by NTF2 and reconverted into RanGTP by RanGEF. Leptomycin B (LMB) inactivates CRM1 by covalent binding to Cys528, thereby irreversibly inhibiting NES-mediated nuclear export. For the selective export of cargoes, NES-specific cofactors (X) have been suggested. RanGEF - Ran guanine nucleotide exchange factor, RanGAP - Ran GTPase activating protein 1, NTF2 - Nuclear Transport Factor 2, CAS - cellular apoptosis susceptibility, X – putative NES-specific cofactor. (b) Heat repeat 10 contains the cysteine residue 528, which is covalently modified by LMB. The C-terminal region (CTR) comprises heat repeats 14 to 19, colored from yellow to black. The CTRs of two proteolytic fragments of CRM1 (residues 707–1027, PDB 1W9C) are displayed as a dimeric solid ribbon representation of the backbone superposition (heat repeats of one fragment colored as above).