Figure 4.
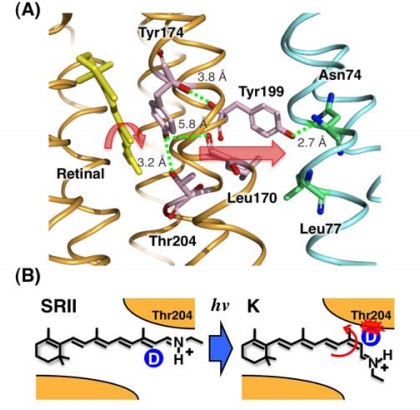
(A) Detail of the SRII-HtrII X-ray structure, which focuses on the midmembrane SRII-HtrII interface containing the core signal relay structure. SRII and HtrII are colored orange and blue, respectively. The numbers in the smaller font are the length between the respective amino acid residues. Using FTIR spectroscopy and photochemical techniques, we and another group reported that Thr199SRII forms a hydrogen bond with Asn74HtrII [56,79]. A functionally important residue, Thr204SRII, forms a hydrogen bond with Tyr174SRII. Tyr174SRII is also essential for the phototaxis function [81]. (B) The structural change of the retinal chromophore upon formation of the K intermediate of SRII. All seven monodeuterated all-trans retinal analogues were synthesized, and the FTIR spectra were measured at 77 K. The enhanced C14-D stretch in the K intermediate was assigned as the band originating from the local steric constraint between C14-D and Thr204SRII [75,80]. We reported that the band intensity correlated well with the phototaxis signaling efficiency, indicating its functional importance [80].