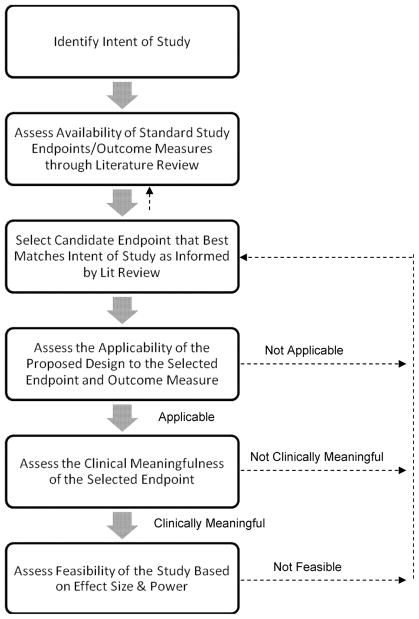
Figure 1. Flow diagram of the steps needed to decide on a primary endpoint for a clinical trial.
For the STRIDE study, the intent of the study is to assess whether or not augmentation of treatment as usual with exercise or health education will improve stimulant use outcomes. Investigators, with the support and guidance of a Protocol Development Team (PDT), reviewed salient literature on treatments for substance use in general, as well as studies that used our specific interventions or that were conducted within our specific population of interest. The team decided upon a primary endpoint of percent days abstinent, as measured by the Time Line Follow Back informed by a daily Substance Use Calendar and urine drug screens collected 3 times per week. The feasibility and applicability of using the TLFB to assess percent days abstinent in a trial of long duration (i.e., 9 months) and in a setting that requires transitioning from residential to outpatient treatment was considered. Consensus was obtained by the PDT regarding the clinical meaningfulness of an endpoint of percent days abstinent. Note that if at any step a problem is encountered (e.g., power analysis reveals that the number of subjects needed to conduct the trial is not feasible, or the effect associated with the selected endpoint is deemed not clinically meaningful), one can return to the previous step(s) to reevaluate the information and select the new endpoint.