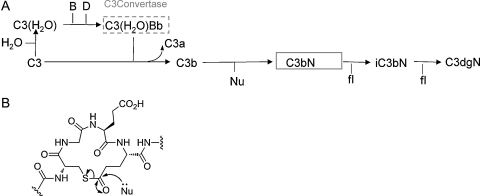
Fig. 1.
Reactivity of the unactivated thioester of complement protein C3. (A) The tick over event of the alternative pathway. C3(H2O) is formed by the slow hydrolysis of C3. Interaction between factor B and C3(H2O) forms an active C3 convertase complex that can generate more C3b molecules by cleavage of the C3a domain from C3, forming a positive feedback loop. C3b has a metastable thioester group that can react rapidly with nucleophiles such as the hydroxyl groups on carbohydrates or amines on proteins. (B) A generalised reaction mechanism for the reaction of a nucleophile with the thioester of full-length C3 (tick over event). This gives a route for the modification of the whole C3 protein without activation. The fifteen-membered intramolecular thiolactone ring is the defining feature of the C3/α-2M thioester protein family, an evolutionary ancient family of proteins that all function by forming covalent linkage of the protein to a target via the thioester bond. The thioester moiety is formed by bridging the cysteine and glutamine side chains with a β-cysteinyl-γ-glutamyl bond.