Abstract
Osteoporosis is a common age-related disorder and causes acute and long-term disability and economic cost. Many factors influence the accumulation of bone minerals, including heredity, diet, physical activity, gender, endocrine functions, and risk factors such as alcohol, drug abuse, some pharmacological drugs or cigarette smoking. The pathology of bone development during intrauterine life is a factor for osteoporosis. Moreover, the placental transfer of nutrients plays an important role in the building of bones of fetuses. The importance of maternal calcium intake and vitamin D status are highlighted in this review. Various environmental factors including nutrition state or maternal stress may affect the epigenetic state of a number of genes during fetal development of bones. Histone modifications as histone hypomethylation, histone hypermethylation, hypoacetylation, etc. are involved in chromatin remodeling, known to contribute to the epigenetic landscape of chromosomes, and play roles in both fetal bone development and osteoporosis. This review will give an overview of epigenetic modulation of bone development and placental transfer of nutrients. In addition, the data from animal and human studies support the role of epigenetic modulation of calcium and vitamin D in the pathogenesis of osteoporosis. We review the evidence suggesting that various genes are involved in regulation of osteoclast formation and differentiation by osteoblasts and stem cells. Epigenetic changes in growth factors as well as cytokines play a rol in fetal bone development. On balance, the data suggest that there is a link between epigenetic changes in placental transfer of nutrients, including calcium and vitamin D, abnormal intrauterine bone development and pathogenesis of osteoporosis.
Keywords: osteoporosis, osteogenesis, epigenomics, vitamin D
Introduction
Osteoporosis is a common age-related disorder. It is characterized by an excessively fragile skeleton and susceptibility to fractures (Harvey et al., 2008). Osteoporosis manifests clinically by fractures of the hip, vertebrae, distal forearm, etc. and the major cause of these fractures is low bone mass. It is well established that the skeletal size and density increase from early embryogenesis through intrauterine, infant, childhood and adult life. Various gene-mapping approaches have been applied to identify specific genes during all periods of bone development. Moreover, genes were detected for osteoporosis, involving largely low bone mineral density (BMD), which is one of the strongest risk factors for osteoporotic fracture. Gene studies focused on BMD and documented the phenotype genetic correlation between BMD and osteoporotic fracture (Bass et al., 1998). Twin and family studies have shown that genetic factors play an important role in osteoporosis. Progress was made in: 1) heritability, osteoporosis and phenotype studies; 2) approaches for gene mapping and identification of gene mutations for osteoporosis; 3) candidate gene association studies; 4) nutrients and osteoporosis; and 5) pharmacological drugs and pharmacogenomic studies for osteoporosis (Long et al., 2005).
Epigenetic regulation of bones and osteoporosis is not well established. Moreover, the effect of nutrition status of the mother has been discussed for many years and several studies considered it as an environmental epigenetic factor that may play an important role during fetal development. Nutrition, especially calcium intake influences the building of bones and prophylaxis of osteoporosis. Mother-offspring studies have demonstrated the role of some specific factors in early life, such as maternal body build, lifestyle and 25(OH)-vitamin D status of the mother, which might be important for bones of the fetus/neonate. In addition, the placenta plays a role in the nutrition status of the developing fetus. It is involved in the transport of nutrients and waste products between mother and fetus, being a source of many peptides and hormones that affect fetal development (Burton et al., 2001). The aim of the present review is to analyze published data in the field of epigenetic regulation of placental transfer of nutrients which may support better prophylaxis and treatment of osteoporosis. This review also summarizes the current knowledge on epigenetics of growth factors, cytokines and hormones in fetal bone development and their impact on osteoporosis.
Basic genetic characteristics of bone development and osteoporosis
Regulatory regions of the human genome can be modified through epigenetic processes during prenatal life. Genetic susceptibility to osteoporosis may be both context dependent and developmentally regulated, and epigenetic mechanisms are the likely link between gene and environment. Epigenetic changes may make individuals more likely to suffer from chronic diseases, as osteoporosis, when they reach adulthood. The modification of chromatin and DNA contributes to a larger well-documented process known as “programing”.
Osteoporosis is one of various well documented “programing” chronic diseases (Tang et al., 2007). Chromosome 11q12-13 has been investigated for its linkage to normal BMD variation (Koller et al., 2000; 2001). Reneland et al.(2005) investigated more than 25,000 single-nucleotide polymorphisms (SNPs) located within 16,000 genes and established that variants in the gene encoding the phosphodiesterase 4D account for some of the genetic contribution to BMD variation in humans. The cause for osteoporosis pseudoglioma is a mutation in LRP5 (low-density lipoprotein receptor-related protein), which is a member of Wnt family genes. Many of the known genes implicated by the 29 loci identified through GWAS participate in the Wnt/β-catenin signaling pathway. It is one of the signaling pathways through which members of the Wnt family can signal (e.g. GPR177, CTNNB1, LRP5, and SFRP4) and regulate bone development and bone loss. Xiong et al.(2009) reported the results from genome-wide association and follow-up replication studies which identified additional genes ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups (Xiong et al., 2009). In addition, the RANKL/RANK/OPG pathway has also emerged from candidate gene and GWAS of BMD (e.g. TNFSF11 and TNFRSF11B). It is known that this pathway is involved in the regulation of osteoclast formation and differentiation by osteoblasts and stromal stem cells. The signaling system that involves receptor activation of nuclear factor-κB (RANK) by its ligand (RANKL), which is regulated by the soluble decoy receptor osteoprotegerin (OPG), plays a role in the RANKL/RANK/OPG pathway of gene control of fetal development of bones and osteoporosis.
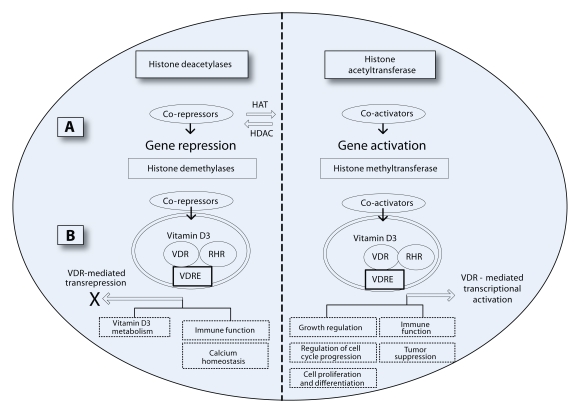
The maintenance of calcium homeostasis by parathyroid hormone (PTH) is well established. Various animal and human studies have shown that the parathyroid gland secretes PTH in response to low serum calcium concentrations, which acts to normalize calcium levels through several mechanisms, including promotion of bone absorption (Nissenson et al., 2009). Some evidence has suggested that genes connected with PTH are strong candidate for genetic regulation of bone development and bone loss. In addition, several studies reported polymorphisms in genes in the PTH pathway. PTH, PTH-like hormone (PTHLH), and PTH1 receptor (PTHR1) are associated with either fracture risk or BMD. Their involvement in fetal bone development is not yet clear (Scillitani et al, 2006; Vilariño-Güell et al., 2007; Gupta et al., 2008). Additional candidate for fetal bone development and osteoporosis is the gene COL1A1 Sp1 for type 1 collagen, which is a major protein of bone. The polymorphism of COL1A1 Sp1 has been associated with osteoporotic fractures and BMD. Moreover, the studies suggest that COL1A1 Sp1 plays a role in fetal bone development (Ralston, 2010). The vitamin D receptor (VDR) gene has been studied for its role in bone metabolism. The studies of Ensrud et al. (2009) evaluated the relation of VDR polymorphisms to bone loss. They found that low 25-hydroxyvitamin D levels have were associated with a higher rate of decline in total hip BMD in men. Several studies have reported associations between polymorphisms in VDR and cross-sectional measures of BMD (Cooper et al., 1996). On balance, the data suggest that the gene for VDR receptor plays a role in fetal bone development. Figure 1 shows the role of VDR gene in regulation of various functions, as cell growth regulation, cell proliferation and differentiation, immune functions, calcium homeostasis, etc., which are important for bones (Sundar and Rahman, 2011). In addition, various transcription factors that play roles in bone formation have been identified. For example, Sox9, Runx2, and Osterix are active in osteoblasts (Baek and Kim, 2011). In addition, the osteogenic genes such as ALP, type I collagen, osteocalcin, and bone sialoprotein (Bsp) are essential for osteoblast differentiation and bone formation (Matsubara et al., 2008).
Figure 1.
Epigenetic mechanism via modifications of histone enzymes (deacetylases, demethylases, acetyltransferase or methyltransferase) plays a role in vitamin D3 (1α,25-dihydroxyvitamin D3)-mediated regulation of cellular and physiological functions.
Histone acetylation correlates with active gene transcription, and deacetylation associated with gene repression. Histone demethylases are involved in vitamin D3 receptor (VDR)-mediated transrepression. Gene repression of vitamin D3 and VDR affects calcium homeostasis, immune function as well as metabolism of vitamin D3. Histone methyltransferase is involved in vitamin D3-mediated cell growth, cell cycle progression, cell proliferation and differentiation, tumor suppression and immune function. VDR-mediated transcriptional activation plays a role in all the above vitamin D3-mediated cellular functions. Histone modifications as histone hypomethylation, histone hypermethylation, histone hypoacetylation, etc. may affect intrauterine growth regulation of bones.
Epigenetics – link between genes and the environment in regulation of bone development and osteoporosis
Epigenetic changes are modifications to the DNA, which can be caused by various factors including environment, and which do not change the underlying sequence. Histone modifications, as histone hypomethylation, histone hypermethylation and hypoacetylation, etc., are involved in chromatin remodeling known to contribute to the epigenetic landscape of chromosomes. Epigenetic modifications can be passed down from the mother or father and can influence even the grandchild generation. They have the capacity to control whether a gene is turned on or off or how much message is processed. These changes to gene expression can lead to dramatic differences in individual characteristics. Disruption of the epigenome is a fundamental mechanism in fetal development diseases. Epigenetic modifications, leading to dysregulation of critical genes controlling cell proliferation, differentiation, and death of cells in fetal bones are not well established. However, epigenetics is a rapidly expanding field and various studies demonstrated the role of epigenetic modifications in bone development and osteoporosis. It has now been shown that the birth weight and childhood growth of 7,000 men and women born in Helsinki University Central Hospital during 1924–1933 were linked to hospital discharge records for hip fracture (Coopere et al., 2001). The results from the Helsinki Cohort Study suggest that the growth in utero and early life of the offspring is associated with the actual risk of fracture. Twin studies also provided various opportunities to study the effect of the environment upon development, while controlling genetic variation (Javaid et al., 2004). In a study of 445 monozygotic and 966 dizygotic twins from the UK, at a mean age of 47 years, birth weight was found to positively predict BMC and BMD (Antoniades et al., 2003). The results suggest that there are epigenetic modifications in the regulation of bone development.
Epigenetics refers to stable and heritable changes in gene expression that do not involve changes in DNA sequence. It is well established that histones stabilize DNA. Many environmental factors interact with genes through epigenetic mechanisms in human diseases, including osteoporosis (Hunter, 2005). Gene-environment interactions are involved in fetal bone development and the changes may cause osteoporosis later in life. A specific genotype may result in a phenotype only in certain environments. For example, the interactions between the genome and fetal environment might establish basal levels of circulating growth hormone and contribute to accelerated bone loss. Many of these interactions occur primarily during early life (in fetuses and neonates). Several environmental factors have been shown to directly influence methylation patterns and expression levels of some genes. Moreover, in some cases, epigenetic alterations are transmissible beyond a single generation (Jiang et al., 2004). Prenatal glucocorticosteroids and neonatal behavioral manipulation (especially stress) promoted changes in histone acetylation and DNA methylation in a transcriptional factor binding site of glucocorticoid receptor (Weaver et al., 2004). The studies cited above on 3,639 men and 3,447 women born in Helsinki University Central Hospital supported the motion of epigenetic regulation of bone development. The results demonstrated that after adjustment for age and gender, there were two major determinants of hip fracture risk: tall maternal height and low rate of childhood growth. The data also showed that the hip fracture risk was elevated among babies born short. Fracture subjects were shorter at birth but of average height by the age of 7 years. These data suggest that the epigenetic mechanisms that respond to in utero or early life exposures play a critical role in growth/development of bones. The studies on animals also support the interactions between environmental factors and epigenetic mechanisms in bone development and osteoporosis. It was shown that alterations to the diet of pregnant animals can produce lasting changes in the offspring's physiology and metabolism (Bateson, 2001). During mammalian development, signals about the nutritional status transferred from the mother to her embryo or fetus either through the placenta or through lactation can affect the epigenetic reprograming in mammals (Morgan et al., 2005). This transfer of signals from mother to fetus may act to limit fetal growth, in preference to any genetic potential, and has been named ‘maternal constraint’ (Gluckman et al., 2008). Poor diet or limited nutrients for the fetus may cause various changes, including epigenetic modifications of histones/genes involved in bone development. In addition, stressful stimuli and cortisol are major factors for epigenetic changes of fetal development. It was documented that the profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men are dependent on fetal growth (Dennison et al., 1999). Moreover, a statistically significant positive association was found between characteristics of cortisol concentration and femoral neck bone loss rate in 22 men, aged 61–72 years, undergoing replicate bone density measurements over a 4-year period. Their data also suggest that the endogenous cortisol profile of healthy elderly men is a determinant of their rate of fetal bone loss.
On balance, the results from human and animal studies suggest that both nutrients and environmental factors as stress, smoking, drug abuse, radiation, etc. may play a role in epigenetic changes of intrauterine bone development.
Placental transfer of nutrients: epigenetic regulations and their role in fetal bone development and osteoporosis
DNA methylation, histone modifications and non-coding RNAs, affect gene expression in the placenta. This expression, including the important parent-of-origin-dependent gene expression resulting from genomic imprinting, plays a role in both fetal and placental development (Nelissen et al., 2011). The study showed that the epigenetic of the placenta is a major factor of placental supply of nutrients to the fetus. Calcium and vitamin D are key nutrients likely to influence fetal bone development. The human fetus requires a total of 30 g of calcium for bone development, most of which is acquired during the third trimester via active transport across the placenta. Fetal calcium needs are primarily met by increased maternal intestinal calcium absorption during pregnancy. The data indicated that very low maternal calcium intake may be a risk for lower bone mass in fetuses as well as in neonates. The importance of maternal vitamin D status has also been studied (Namgung and Tsang, 2003). It is well established that 1,25(OH)-vitamin D (the active form) mediates vitamin D effects first by binding to the vitamin D receptors (VDR), then by binding to the retinoic acid receptor (RXR), forming a heterodimer (Kimball et al., 2008). This heterodimer then acts upon vitamin D response elements in target genes, initiating gene transcription by either up-regulating or down-regulating gene products. The expression of a placental calcium transporter (PMCA3) gene predicts neonatal whole-body BMC (Martin et al., 2007). The effects of maternal nutrition target the promoter region of specific genes. The VDR, the collagen type I alpha 1 gene (COLIA1) and estrogen receptor gene (ER) alpha have been most widely investigated and found to play a role in regulating BMD as well as targets for epigenetic regulation of placental transfer of nutrients (Williams and Spector, 2006). Epigenetic changes may associate with changes in DNA methylation of genes within or located near to vitamin D response elements. Modified expression of the genes encoding placental calcium transporters by epigenetic regulation may indicates data that maternal vitamin D status influences bone mineral homeostasis in the neonate. WNT2 promoter methylation in the human placenta was found to be associated with low birth weight percentile in the neonate (Ferreira et al., 2011).
The DNA methylation differences identified in the promoter of the WNT2 gene were studied in an extended cohort of 170 samples. The results demonstrated that the WNT2 gene plays an important role in mouse placental development and confirmed its high expression in the human placenta. High WNT2 promoter methylation (WNT2PrMe) was found only in placental tissue and not in the cord blood of the fetus. The results also showed that WNT2 expression can be epigenetically down-regulated in the placenta by DNA methylation of its promoter and that high WNT2PrMe is an epigenetic variant associated with reduced fetal growth potential. Dysregulation of the genes led to the hypothesis that various epigenetic changes in the placenta responsible for nutrients transfer to the fetus played roles in abnormal fetal bone development and contributed to osteoporosis in the adults. Moreover, the results support the view that low birth weight as well as low BMD may result from maternal, fetal, placental and environmental factors.
In addition, various studies suggest that the epigenetic modulation of the HPA axis represents a second mechanism by which poor maternal nutrition as well as environment may cause epigenetic changes in bones of fetuses and neonates. Protein restriction during mid and late pregnancy was found to be associated with reduced methylation of key CpG-rich islands in the promoter region of the gene for the glucocorticoid recpetor (GR) (Weaver et al., 2004). Further there was increased expression of the GR and DNA methylation, which is maintained through mitosis by DNA methyltransferase-1 (Dnmt1) activity. Moreover, the phenotype of an embryo can be modified by manipulation of DNA methyltransferase-1 (Dnmt1) expression by DNA methylation (Biniszkiewicz et al., 2002; Bird, 2001). In addition, several studies have confirmed that independent predictors of greater neonatal whole-body bone area and BMC include genes and their role in regulation of greater maternal birth weight, height and fat stores. Maternal activities such as smoking or drug abuse, dietary choices including calcium and vitamin D intake, and endocrine functions are factors for offspring bone development and may play roles in epigenetic mechanisms of placental transport of nutrients (Dennison et al., 1999, 2004; Javaid et al., 2004, 2005). Thus, for example, data that maternal smoking is statistically significantly associated with lower neonatal bone mass suggest a possible role of epigenetic changes.
Epigenetic pathways in bone development in animal and human models
Rat dietary protein restriction leads to altered Dnmt1 expression. Dnmt1 expression in human umbilical cord predicts GR1-Ctotal (human glucocorticoid receptor 1-Ctotal promoter) methylation. It was established that GR1-C total methylation status in human umbilical cord predicts GR expression. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications (Lillycrop et al., 2007). Cooney et al. (2002) found that maternal methyl supplements in mice affected epigenetic variation and DNA methylation of the offspring. In the agouti mouse mutant, maternal dietary folate supplementation at conception altered the expression of the imprinted agouti gene by altering the capacity for methylation (Cooney et al., 2002). The relationship between poor nutrition in utero, weight in infancy and adult BMD has been replicated in population studies in the United States, Australia and Scandinavia (Cooper et al., 2006). These results suggest that genetic or epigenetic influences on bone development may be modified by poor nutrition in utero. Studies in 198 children aged 9 years demonstrated that lower preconception maternal weight, reduced maternal fat stores during late pregnancy, a history of maternal smoking during pregnancy and lower maternal social class were all associated with reduced whole-body BMC of the child at the age of 9 years (Javaid et al., 2006). Moreover, a lower ionized calcium concentration in umbilical venous serum may predict reduced fetus and neonate bone mass. Around 31% of the mothers had insufficient and 18% had deficient circulating concentrations of 25(OH)-vitamin D during late pregnancy. Lower concentrations of serum 25(OH)-vitamin D in mothers during late pregnancy were associated with reduced whole-body and lumbar spine BMC in children at the age of 9 years.
Growth factors (GF) play important roles in bone development (Winsloe et al., 2009). IGF-2 is known to be a key factor in human growth and development and it was found to be associated with epigenetic changes Interestingly, IGF-2 gene was hypomethylated, whereas interleukin-10, leptin, ATP-binding cassette A1 and maternally expressed 3 (meg 3) genes were hypermethylated. This study further supports the importance of investigating how early epigenetic modification of gene expression may influence long-term health and disease.
IGF2 and H19, belonging to the same cluster of imprinted genes and regulated by ICR1, DMR2 and H19 promoter elements, play a major role in fetal/placental growth. The epigenetic modulation of IGF2/H19 during human development was marked in 60 normal and 66 idiopathic IUGR (Intrauterine Growth Restriction) pregnancies. Embryonic (cord blood) and extraembryonic (placenta and umbilical cord) tissues were studied. Normal ICR1 methylation levels (approximately 50%) and H19 promoter/DMR2 hypomethylation were found in extra-embryonic tissues. In contrast, in embryonic samples the three loci displayed normal methylation values comparable to those in postnatal blood. Asymmetric allelic expression of H19 and IGF2 was reported as a common feature in pre- and post-natal tissues, independent of H19 promoter and DMR2 methylation levels. The study suggests that the gradient of global methylation, increasing from extra-embryonic to embryonic and adult tissues, might play an important role in fetal/bone development. In addition, hypomethylation of H19 promoter and DMR2 were not found to influence the expression pattern of IGF2 and H19 (Tabano et al., 2011).
The role of cytokines in epigenetic regulation of bones is still not well established. There are data indicating that IL-6 genetic variation is prominently associated with hip BMD in late postmenopausal women without estrogen replacement therapy and/or with inadequate calcium intake (Ferrari et al., 2004). Growth factors interact with various cytokines in regulation of fetal development and further studies will determine whether the epigenetic modulations play roles in GF/cytokine relations.
Conclusion
Osteoporosis is a complex disease determined by genetic and environmental factors, as well as possible interactions among these factors. Epigenetic regulation of placental transfer of nutrients plays a role in both bone development and osteoporosis. Hypermethylation or hypomethylation, hypoacetylation or hyperacetylation of genes were found to regulate calcium, vitamin D, cytokines, growth factors, etc. in bone development.
REFERENCES
- Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology. 2003;42:791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- Baek WY, Kim JE. Transcriptional regulation of bone formation. Front Biosci. 2011;3:126–135. doi: 10.2741/s138. [DOI] [PubMed] [Google Scholar]
- Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–507. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- Bateson P. Fetal experience and good adult design. Int J Epidemiol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- Biniszkiewicz D, Gribnau J, Ramsahoye B, et al. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2213. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2001;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester – a review. Placenta. 2001;22(Suppl. A):S70–S77. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393–2400. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Umbach DM. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J Bone Miner Res. 1996;11:1841–1849. doi: 10.1002/jbmr.5650111203. [DOI] [PubMed] [Google Scholar]
- Coopere C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJP. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporosis Int. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- Dennison EM, Syddall HE, Fall CH, Javaid MK, Arden NK, Phillips DIW, Cooper C. Plasma Leptin Concentration and Change in Bone Density among Elderly Men and Women: The Hertfordshire Cohort Study. Calcif Tissue Int. 2004;74:401–406. doi: 10.1007/s00223-002-0017-x. [DOI] [PubMed] [Google Scholar]
- Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, Cooper C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab. 1999;84:3058–3063. doi: 10.1210/jcem.84.9.5964. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Taylor BC, Paudel ML. Osteoporotic Fractures in Men Study Group.(2009). Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Karasik D, Liu J, Karamohamed S, Herbert AG, Cupples LA, Kiel DP. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham Osteoporosis Study. J Bone Miner Res. 2004;19:552–559. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- Ferreira JC, Choufani S, Grafodatskaya D, Butcher DT, Zhao C, et al. WNT2 promoter methylation in human placenta is associated with low birth weight percentile in the neonate. Epigenetics. 2011;6:440–449. doi: 10.4161/epi.6.4.14554. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Eng J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Välimäki VV, Välimäki M, Löyttyniemi E, et al. Variable number of tandem repeats polymorphism in parathyroid hormone-related protein as predictor of peak bone mass in young healthy Finnish males. Eur J Endocrinol. 2008;158:755–764. doi: 10.1530/EJE-07-0886. [DOI] [PubMed] [Google Scholar]
- Harvey N, Dennison E, Cooper C. Epidemiology of osteoporotic fracture. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism”. 7th edn. Washington: ASMBR; 2008. pp. 198–203. [Google Scholar]
- Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Godfrey KM, Taylor P, Robinson SM, Crozier SR, et al. Umbilical cord leptin predicts neonatal bone mass. Calcif Tissue Int. 2005;76:341–347. doi: 10.1007/s00223-004-1128-3. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Godfrey KM, Taylor P, Shore SR, Breier B, Arden NK, Cooper C. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J Bone Miner Res. 2004;19:56–63. doi: 10.1359/JBMR.0301211. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Bressler J, Beaudet AL. Epigenetics and human disease. Annu Rev Genomics Hum Genet. 2004;5:479–510. doi: 10.1146/annurev.genom.5.061903.180014. [DOI] [PubMed] [Google Scholar]
- Kimball S, El-Hajj Fuleihan G, Vieth R. Vitamin D: a growing perspective. Clinical Laboratory Sciences. 2008;45:339–414. doi: 10.1080/10408360802165295. [DOI] [PubMed] [Google Scholar]
- Koller DL, Liu G, Econs MJ, Morin P, Christian JC, et al. Genome screen for quantities trail loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;15(Suppl.1):1094. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- Koller DL, Econs M J, Rodríguez LA, Christian JC, et al. Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab. 2000;85:3116–3120. doi: 10.1210/jcem.85.9.6778. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Slater-Jefferies JL, Hanson MA, et al. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JR, Xiong DH, Recker RR, Deng HW. The genetics of osteoporosis. Drugs Today (Barc) 2005;41:205–218. doi: 10.1358/dot.2005.41.3.892525. [DOI] [PubMed] [Google Scholar]
- Martin R, Harvey NC, Crozier SR, et al. Placental calcium transporter (PMCA3) gene expression predicts intrauterine bone mineral accrual. Bone. 2007;40:1203–1208. doi: 10.1016/j.bone.2006.12.060. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, et al. BMP2 regulates osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14:47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- Namgung R, Tsang RC. Bone in the pregnant mother and newborn at birth. Clin Chim Acta. 2003;333:1–11. doi: 10.1016/s0009-8981(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum Reprod Update. 2011;17:397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- Nissenson RA, Jüppner H. Primer on the metabolic bone diseases and disorders of mineral metabolism. Hoboken, (NJ): John Wiley & Sons; 2009. Parathyroid hormone; pp. 123–127. [Google Scholar]
- Ralston SH. Genetics of osteoporosis. Ann NY Acad Sci. 2010;1192:181–189. doi: 10.1111/j.1749-6632.2009.05317.x. [DOI] [PubMed] [Google Scholar]
- Reneland RH, Mah S, Kammerer S, Hoyal CR, Marnellos G, Wilson SG, Sambrook PN, et al. Association between a variation in the phosphodiesterase 4D gene and bone mineral density. BMC Med Genet. 2005;6:9. doi: 10.1186/1471-2350-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scillitani A, Jang C, Wong BY, Hendy GN, Cole DE, et al. A functional polymorphism in the PTHR1 promoter region is associated with adult height and BMD measured at the femoral neck in a large cohort of young caucasian women. Hum Genet. 2006;119:416–421. doi: 10.1007/s00439-006-0155-8. [DOI] [PubMed] [Google Scholar]
- Sundar IK, Rahman I. Vitamin D and susceptibility of chronic lung diseases: role of epigenetics. Front Pharmacology. 2011;2:1–10. doi: 10.3389/fphar.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabano S, Colapietro P, Cetin I, Grati FR, Zanutto S, et al. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2011;16:313–24. doi: 10.4161/epi.5.4.11637. [DOI] [PubMed] [Google Scholar]
- Tang W, Ho S. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–182. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C, Miles LJ, Duncan EL, Ralston SH. PTHR1 polymorphisms influence BMD variation through effects on the growing skeleton. Calcif Tissue Int. 2007;81:270–278. doi: 10.1007/s00223-007-9072-7. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Williams FM, Spector TD. Recent advances in the genetics of osteoporosis. J Musculoskelet Neuronal Interact. 2006;6:27–35. [PubMed] [Google Scholar]
- Winsloe C, Earl S, Dennison EM, Cooper C, Harvey NC. Early life factors in the pathogenesis of osteoporosis. Curr Osteoporos Rep. 2009;7:140–144. doi: 10.1007/s11914-009-0024-1. [DOI] [PubMed] [Google Scholar]
- Xiong DH, Liu XG, Guo YF, Tan LJ, Wang L, Sha BY, Tang ZH. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet. 2009;84:388–398. doi: 10.1016/j.ajhg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]