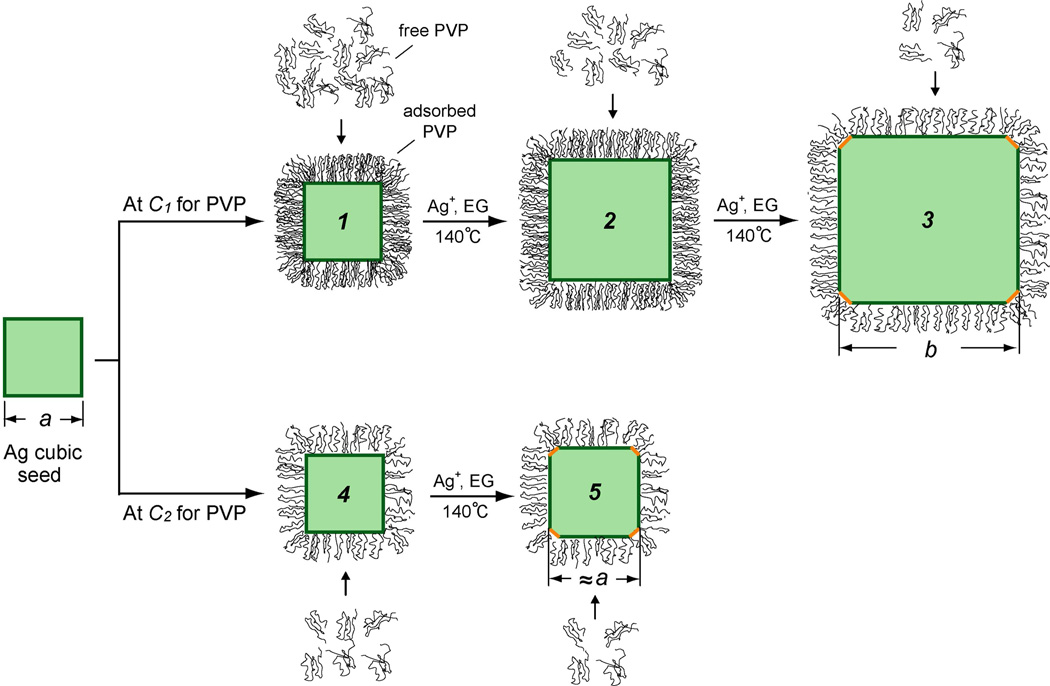
Figure 1.
A schematic showing the growth of a Ag cubic seed with an edge length of a nm in the presence of PVP at a high concentration of C1 and a critical concentration of C2 (C1>C2), respectively. At C1, enough free PVP in the solution ensures surface adsorption and thus good passivation of the Ag(100) surface of the cubic seed (1), which can grow to a bigger cube (2), accompanied by reduction in PVP concentration due to its adsorption onto the newly formed Ag surface. Eventually, when the concentration of free PVP drops to a critical value (C2), the PVP can no longer passivate the Ag(100) surface, the free energies of {100} and {111} facets become the same, and {111} facets start to appear on the surface, leading to the formation a larger cube of b nm in size (3) with slight truncation at corners. Alternatively, when the initial concentration of PVP is reduced to the critical value (C2), {111} facets start to appear on the surface of the cubic seed at the very beginning of growth, leading to the formation of a slightly truncated cube (5) with essentially the same size as the initial seed.