Table 4.
FAK Inhibitors
| Inhibitor Name | Chemical Name | Structure | |
|---|---|---|---|
| PF 573,228 (PF-228) | 6-(4-(3-(methylsulfonyl) benzylamino)-5- (trifluoromethyl)pyrimidin- 2-ylamino)-3, 4-dihydro quinolin-2(1H)-one |
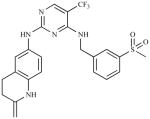
|
Pfizer |
| PF562, 271-26 (PF-271) | N-Methyl-N-(3- {[2-(2-oxo-2,3-dihydro-1H-indol- 5-ylamino)-5-trifluoromethyl- pyrimidin-4-ylamino]-methyl}- pyridin-2-yl)-methanesulfonamide |
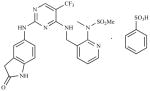
|
Pfizer |
| NVP-226 | (2-[5-Chloro-2-[2-methoxy-4- (4-morpholinyl)phenylamino] pyrimidin-4-ylamino]-N- methylbenzamide |
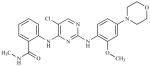
|
Novartis |
| Y15* | 1,2,4,5-Benzenetetraamine tetrahydrochloride |
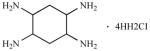 · 4HH2CI |
|
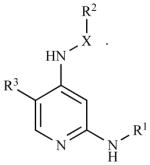
| PND-1186** | 2,4-diamino-pyridine-based; X=bond or (C1-C3)alkyl comprising 0–1 heteroatom selected from the group consisting of N, O, S(O) and S(O)2, the (C1-C3)alkyl is substituted with 0–1 hydroxy, halo, (C1-C3)alkoxy, (C1-C3)alkylamino or (C1-C3)2dialkylamino groups. R1 and R2 are 5–12 membered Mono-, bi- or polycyclic, aromatic or partially aromatic rings. R3 is a trifluoromethyl, halo, nitro or cyano; salt, tautomer, solvate, hydrate, or aprodrug. |
|
Reported by Golubovskaya et al., J. Med. Chem, V 51, p7405–7416, 2008, ref. in (15).
Reported by Tanjoni et al., Cancer Biol. Ther., 2010, V 15, 2010, ref in (17).
