Abstract
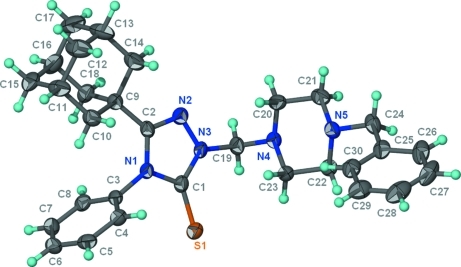
The title molecule, C30H37N5S, displays a chair-shaped piperazine ring, as well as an approximately planar triazole ring [maximum deviation = 0.002 (2) Å] whose phenyl substituent is nearly perpendicular to the mean plane of the five-membered ring [dihedral angle = 80.4 (1)°]. The substituents on the piperazine ring occupy equatorial sites. Weak intermolecular C—H⋯S hydrogen bonding is present in the crystal structure.
Related literature
For background to 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives, see: El-Emam & Ibrahim (1991 ▶).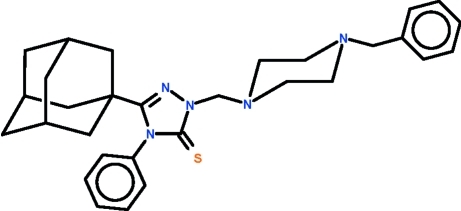
Experimental
Crystal data
C30H37N5S
M r = 499.71
Triclinic,
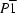
a = 10.1677 (6) Å
b = 11.3287 (7) Å
c = 12.5331 (7) Å
α = 67.037 (6)°
β = 85.768 (5)°
γ = 83.547 (5)°
V = 1320.12 (13) Å3
Z = 2
Mo Kα radiation
μ = 0.15 mm−1
T = 100 K
0.15 × 0.15 × 0.05 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010 ▶) T min = 0.978, T max = 0.993
9288 measured reflections
6033 independent reflections
4002 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.151
S = 1.01
6033 reflections
325 parameters
H-atom parameters constrained
Δρmax = 0.76 e Å−3
Δρmin = −0.39 e Å−3
Data collection: CrysAlis PRO (Agilent, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681105570X/xu5419sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681105570X/xu5419Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681105570X/xu5419Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C13—H13⋯S1i | 1.00 | 2.85 | 3.751 (3) | 150 |
| C28—H28⋯S1ii | 0.95 | 2.84 | 3.673 (4) | 146 |
Symmetry codes: (i) 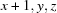 ; (ii)
; (ii) 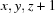 .
.
Acknowledgments
We thank the Deanship of Scientific Research and the Research Center of the College of Pharmacy, King Saud University, and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
We reported the synthesis, anti-inflammatory and analgesic properties of 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives (El-Emam & Ibrahim, 1991). The triazole ring, which possesses a secondary nitrogen site next to a double-bond sulfur, is capable of undergoing a Mannich reaction with an N-substituted piperazine derivative to yield a new class of chemotherapeutic compounds. The C30H37N5S molecule (Scheme I, Fig. 1) displays a chair-shaped piperazine ring, as well as a planar triazole ring whose phenyl substituent is nearly perpendicular to the mean plane of the five-membered ring (dihedral angle 80.4 (1)°).
Experimental
5-(1-Adamantyl)-4-phenyl-1,2,4-triazole-3-thiol was synthesized according to a reported procedure (El-Emam & Ibrahim, 1991). The compound (2 mmol), 1-benzylpiperazine (2 mmol) and a 37% formaldehyde solution (0.5 ml) in ethanol (8 ml), was heated for 15 minutes. Stirring was continued for 12 h at room temperature. The product was filtered, washed with water, dried, and recrystallized from ethanol to yield (80%) of the title compound as colorless crystals, m.p. 470–472 K.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C–H 0.95 to 1.00 Å, Uiso(H) 1.2 to 1.5Ueq(C)] and were included in the refinement in the riding model approximation.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C30H37N5S at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C30H37N5S | Z = 2 |
| Mr = 499.71 | F(000) = 536 |
| Triclinic, P1 | Dx = 1.257 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.1677 (6) Å | Cell parameters from 2374 reflections |
| b = 11.3287 (7) Å | θ = 2.6–27.5° |
| c = 12.5331 (7) Å | µ = 0.15 mm−1 |
| α = 67.037 (6)° | T = 100 K |
| β = 85.768 (5)° | Irregular, colorless |
| γ = 83.547 (5)° | 0.15 × 0.15 × 0.05 mm |
| V = 1320.12 (13) Å3 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 6033 independent reflections |
| Radiation source: SuperNova (Mo) X-ray Source | 4002 reflections with I > 2σ(I) |
| Mirror | Rint = 0.035 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 27.6°, θmin = 2.6° |
| ω scan | h = −13→13 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010) | k = −11→14 |
| Tmin = 0.978, Tmax = 0.993 | l = −13→16 |
| 9288 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.151 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0475P)2 + 0.7934P] where P = (Fo2 + 2Fc2)/3 |
| 6033 reflections | (Δ/σ)max = 0.001 |
| 325 parameters | Δρmax = 0.76 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.03136 (7) | 0.21542 (6) | 0.47877 (6) | 0.03032 (19) | |
| N1 | 0.2538 (2) | 0.34089 (19) | 0.38748 (16) | 0.0234 (5) | |
| N2 | 0.4183 (2) | 0.1937 (2) | 0.46788 (17) | 0.0288 (5) | |
| N3 | 0.2963 (2) | 0.1453 (2) | 0.50537 (17) | 0.0266 (5) | |
| N4 | 0.3003 (2) | −0.0352 (2) | 0.69837 (17) | 0.0248 (5) | |
| N5 | 0.3255 (2) | −0.0357 (2) | 0.92328 (17) | 0.0267 (5) | |
| C1 | 0.1931 (2) | 0.2324 (2) | 0.4584 (2) | 0.0244 (6) | |
| C2 | 0.3904 (2) | 0.3133 (2) | 0.3963 (2) | 0.0241 (6) | |
| C3 | 0.1796 (2) | 0.4566 (2) | 0.3133 (2) | 0.0230 (5) | |
| C4 | 0.1219 (2) | 0.5441 (2) | 0.3584 (2) | 0.0264 (6) | |
| H4 | 0.1265 | 0.5264 | 0.4387 | 0.032* | |
| C5 | 0.0571 (2) | 0.6581 (2) | 0.2848 (2) | 0.0296 (6) | |
| H5 | 0.0174 | 0.7196 | 0.3144 | 0.036* | |
| C6 | 0.0503 (3) | 0.6822 (3) | 0.1679 (2) | 0.0318 (6) | |
| H6 | 0.0076 | 0.7612 | 0.1173 | 0.038* | |
| C7 | 0.1052 (3) | 0.5919 (3) | 0.1245 (2) | 0.0307 (6) | |
| H7 | 0.0989 | 0.6084 | 0.0446 | 0.037* | |
| C8 | 0.1691 (2) | 0.4778 (2) | 0.1973 (2) | 0.0251 (6) | |
| H8 | 0.2054 | 0.4146 | 0.1684 | 0.030* | |
| C9 | 0.4971 (2) | 0.3991 (2) | 0.3296 (2) | 0.0252 (6) | |
| C10 | 0.4604 (3) | 0.5433 (2) | 0.3052 (2) | 0.0283 (6) | |
| H10A | 0.3785 | 0.5737 | 0.2604 | 0.034* | |
| H10B | 0.4433 | 0.5550 | 0.3794 | 0.034* | |
| C11 | 0.5733 (3) | 0.6229 (3) | 0.2364 (3) | 0.0354 (7) | |
| H11 | 0.5476 | 0.7159 | 0.2204 | 0.043* | |
| C12 | 0.6989 (3) | 0.5787 (3) | 0.3071 (3) | 0.0475 (8) | |
| H12A | 0.7716 | 0.6305 | 0.2633 | 0.057* | |
| H12B | 0.6827 | 0.5913 | 0.3810 | 0.057* | |
| C13 | 0.7381 (3) | 0.4360 (3) | 0.3320 (3) | 0.0444 (8) | |
| H13 | 0.8199 | 0.4068 | 0.3784 | 0.053* | |
| C14 | 0.6254 (3) | 0.3553 (3) | 0.4001 (2) | 0.0359 (7) | |
| H14A | 0.6086 | 0.3653 | 0.4751 | 0.043* | |
| H14B | 0.6515 | 0.2633 | 0.4164 | 0.043* | |
| C15 | 0.5980 (3) | 0.6050 (3) | 0.1216 (2) | 0.0402 (7) | |
| H15A | 0.6685 | 0.6586 | 0.0752 | 0.048* | |
| H15B | 0.5163 | 0.6329 | 0.0766 | 0.048* | |
| C16 | 0.6400 (3) | 0.4628 (3) | 0.1454 (3) | 0.0392 (7) | |
| H16 | 0.6575 | 0.4516 | 0.0702 | 0.047* | |
| C17 | 0.7653 (3) | 0.4186 (3) | 0.2161 (3) | 0.0484 (9) | |
| H17A | 0.8383 | 0.4700 | 0.1720 | 0.058* | |
| H17B | 0.7923 | 0.3270 | 0.2311 | 0.058* | |
| C18 | 0.5277 (3) | 0.3819 (3) | 0.2139 (2) | 0.0292 (6) | |
| H18A | 0.5536 | 0.2900 | 0.2295 | 0.035* | |
| H18B | 0.4472 | 0.4087 | 0.1672 | 0.035* | |
| C19 | 0.2901 (3) | 0.0056 (2) | 0.5756 (2) | 0.0273 (6) | |
| H19A | 0.2053 | −0.0195 | 0.5606 | 0.033* | |
| H19B | 0.3623 | −0.0424 | 0.5475 | 0.033* | |
| C20 | 0.4283 (2) | −0.0210 (3) | 0.7366 (2) | 0.0284 (6) | |
| H20A | 0.4393 | 0.0713 | 0.7148 | 0.034* | |
| H20B | 0.5011 | −0.0578 | 0.6987 | 0.034* | |
| C21 | 0.4328 (3) | −0.0913 (3) | 0.8676 (2) | 0.0301 (6) | |
| H21A | 0.4232 | −0.1838 | 0.8890 | 0.036* | |
| H21B | 0.5193 | −0.0836 | 0.8946 | 0.036* | |
| C22 | 0.1978 (2) | −0.0506 (3) | 0.8851 (2) | 0.0269 (6) | |
| H22A | 0.1249 | −0.0140 | 0.9231 | 0.032* | |
| H22B | 0.1872 | −0.1431 | 0.9076 | 0.032* | |
| C23 | 0.1912 (2) | 0.0185 (2) | 0.7543 (2) | 0.0263 (6) | |
| H23A | 0.1054 | 0.0078 | 0.7282 | 0.032* | |
| H23B | 0.1980 | 0.1116 | 0.7320 | 0.032* | |
| C24 | 0.3334 (3) | −0.0941 (3) | 1.0496 (2) | 0.0312 (6) | |
| H24A | 0.4273 | −0.1060 | 1.0706 | 0.037* | |
| H24B | 0.2998 | −0.1802 | 1.0783 | 0.037* | |
| C25 | 0.2551 (3) | −0.0143 (3) | 1.1093 (2) | 0.0302 (6) | |
| C26 | 0.2347 (3) | −0.0657 (3) | 1.2300 (2) | 0.0394 (7) | |
| H26 | 0.2647 | −0.1529 | 1.2735 | 0.047* | |
| C27 | 0.1715 (3) | 0.0091 (4) | 1.2860 (3) | 0.0466 (9) | |
| H27 | 0.1591 | −0.0270 | 1.3679 | 0.056* | |
| C28 | 0.1259 (3) | 0.1364 (4) | 1.2242 (3) | 0.0458 (8) | |
| H28 | 0.0818 | 0.1873 | 1.2633 | 0.055* | |
| C29 | 0.1449 (3) | 0.1876 (3) | 1.1068 (2) | 0.0376 (7) | |
| H29 | 0.1141 | 0.2747 | 1.0640 | 0.045* | |
| C30 | 0.2092 (3) | 0.1134 (3) | 1.0491 (2) | 0.0304 (6) | |
| H30 | 0.2219 | 0.1507 | 0.9672 | 0.036* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0259 (4) | 0.0258 (4) | 0.0312 (4) | −0.0016 (3) | 0.0045 (3) | −0.0034 (3) |
| N1 | 0.0239 (11) | 0.0159 (10) | 0.0236 (11) | 0.0017 (9) | 0.0038 (8) | −0.0019 (8) |
| N2 | 0.0271 (12) | 0.0223 (12) | 0.0291 (12) | 0.0024 (9) | 0.0050 (9) | −0.0036 (9) |
| N3 | 0.0258 (12) | 0.0199 (11) | 0.0244 (11) | 0.0011 (9) | 0.0052 (9) | 0.0002 (8) |
| N4 | 0.0250 (11) | 0.0218 (11) | 0.0231 (11) | −0.0007 (9) | −0.0002 (8) | −0.0042 (8) |
| N5 | 0.0267 (12) | 0.0239 (12) | 0.0255 (11) | −0.0020 (9) | −0.0037 (9) | −0.0047 (9) |
| C1 | 0.0274 (14) | 0.0196 (13) | 0.0220 (13) | 0.0004 (10) | 0.0033 (10) | −0.0049 (10) |
| C2 | 0.0276 (14) | 0.0196 (13) | 0.0207 (12) | 0.0035 (11) | 0.0003 (10) | −0.0045 (10) |
| C3 | 0.0204 (13) | 0.0156 (12) | 0.0265 (13) | −0.0018 (10) | 0.0009 (10) | −0.0013 (10) |
| C4 | 0.0241 (14) | 0.0255 (14) | 0.0272 (13) | −0.0030 (11) | 0.0023 (10) | −0.0080 (11) |
| C5 | 0.0205 (13) | 0.0231 (14) | 0.0424 (16) | −0.0017 (11) | 0.0020 (11) | −0.0103 (12) |
| C6 | 0.0240 (14) | 0.0210 (14) | 0.0383 (16) | −0.0002 (11) | −0.0025 (11) | 0.0016 (11) |
| C7 | 0.0272 (14) | 0.0304 (15) | 0.0269 (14) | −0.0069 (12) | −0.0034 (11) | −0.0014 (11) |
| C8 | 0.0243 (13) | 0.0225 (13) | 0.0274 (13) | −0.0069 (11) | 0.0030 (10) | −0.0079 (10) |
| C9 | 0.0244 (13) | 0.0215 (13) | 0.0253 (13) | 0.0011 (11) | 0.0007 (10) | −0.0054 (10) |
| C10 | 0.0259 (14) | 0.0211 (13) | 0.0353 (15) | 0.0006 (11) | −0.0033 (11) | −0.0085 (11) |
| C11 | 0.0243 (15) | 0.0232 (14) | 0.0557 (18) | −0.0027 (11) | −0.0055 (12) | −0.0111 (13) |
| C12 | 0.0303 (17) | 0.0334 (18) | 0.077 (2) | −0.0034 (13) | −0.0110 (15) | −0.0177 (16) |
| C13 | 0.0258 (16) | 0.0371 (18) | 0.065 (2) | 0.0036 (13) | −0.0158 (14) | −0.0134 (15) |
| C14 | 0.0317 (16) | 0.0301 (16) | 0.0410 (16) | 0.0061 (13) | −0.0090 (12) | −0.0095 (12) |
| C15 | 0.0304 (16) | 0.0332 (17) | 0.0441 (17) | −0.0103 (13) | 0.0074 (13) | −0.0004 (13) |
| C16 | 0.0340 (16) | 0.0376 (17) | 0.0411 (17) | −0.0087 (13) | 0.0128 (13) | −0.0110 (13) |
| C17 | 0.0266 (16) | 0.0361 (18) | 0.076 (2) | −0.0025 (14) | 0.0127 (15) | −0.0165 (16) |
| C18 | 0.0298 (15) | 0.0251 (14) | 0.0302 (14) | −0.0057 (11) | 0.0057 (11) | −0.0082 (11) |
| C19 | 0.0310 (15) | 0.0171 (13) | 0.0264 (13) | 0.0028 (11) | 0.0024 (11) | −0.0024 (10) |
| C20 | 0.0213 (13) | 0.0231 (14) | 0.0354 (15) | 0.0000 (11) | 0.0008 (11) | −0.0064 (11) |
| C21 | 0.0254 (14) | 0.0243 (14) | 0.0381 (15) | 0.0003 (11) | −0.0051 (11) | −0.0092 (11) |
| C22 | 0.0248 (14) | 0.0258 (14) | 0.0277 (14) | −0.0050 (11) | −0.0016 (10) | −0.0069 (11) |
| C23 | 0.0215 (13) | 0.0261 (14) | 0.0264 (13) | −0.0022 (11) | 0.0001 (10) | −0.0050 (10) |
| C24 | 0.0376 (16) | 0.0224 (14) | 0.0284 (14) | −0.0054 (12) | −0.0082 (11) | −0.0022 (11) |
| C25 | 0.0299 (15) | 0.0330 (16) | 0.0275 (14) | −0.0146 (12) | −0.0025 (11) | −0.0080 (11) |
| C26 | 0.0472 (18) | 0.0422 (18) | 0.0277 (15) | −0.0247 (15) | −0.0038 (13) | −0.0062 (13) |
| C27 | 0.048 (2) | 0.070 (2) | 0.0283 (16) | −0.0365 (18) | 0.0087 (13) | −0.0200 (16) |
| C28 | 0.0383 (18) | 0.064 (2) | 0.0495 (19) | −0.0237 (16) | 0.0098 (14) | −0.0349 (17) |
| C29 | 0.0314 (16) | 0.0454 (18) | 0.0431 (17) | −0.0077 (13) | 0.0013 (13) | −0.0241 (14) |
| C30 | 0.0309 (15) | 0.0328 (15) | 0.0292 (14) | −0.0082 (12) | 0.0008 (11) | −0.0127 (12) |
Geometric parameters (Å, °)
| S1—C1 | 1.667 (3) | C13—H13 | 1.0000 |
| N1—C1 | 1.385 (3) | C14—H14A | 0.9900 |
| N1—C2 | 1.391 (3) | C14—H14B | 0.9900 |
| N1—C3 | 1.443 (3) | C15—C16 | 1.535 (4) |
| N2—C2 | 1.313 (3) | C15—H15A | 0.9900 |
| N2—N3 | 1.388 (3) | C15—H15B | 0.9900 |
| N3—C1 | 1.351 (3) | C16—C17 | 1.528 (4) |
| N3—C19 | 1.487 (3) | C16—C18 | 1.536 (4) |
| N4—C19 | 1.432 (3) | C16—H16 | 1.0000 |
| N4—C20 | 1.465 (3) | C17—H17A | 0.9900 |
| N4—C23 | 1.469 (3) | C17—H17B | 0.9900 |
| N5—C22 | 1.466 (3) | C18—H18A | 0.9900 |
| N5—C21 | 1.467 (3) | C18—H18B | 0.9900 |
| N5—C24 | 1.463 (3) | C19—H19A | 0.9900 |
| C2—C9 | 1.513 (3) | C19—H19B | 0.9900 |
| C3—C4 | 1.380 (3) | C20—C21 | 1.521 (3) |
| C3—C8 | 1.388 (3) | C20—H20A | 0.9900 |
| C4—C5 | 1.387 (3) | C20—H20B | 0.9900 |
| C4—H4 | 0.9500 | C21—H21A | 0.9900 |
| C5—C6 | 1.387 (4) | C21—H21B | 0.9900 |
| C5—H5 | 0.9500 | C22—C23 | 1.519 (3) |
| C6—C7 | 1.384 (4) | C22—H22A | 0.9900 |
| C6—H6 | 0.9500 | C22—H22B | 0.9900 |
| C7—C8 | 1.380 (3) | C23—H23A | 0.9900 |
| C7—H7 | 0.9500 | C23—H23B | 0.9900 |
| C8—H8 | 0.9500 | C24—C25 | 1.513 (4) |
| C9—C18 | 1.542 (3) | C24—H24A | 0.9900 |
| C9—C10 | 1.547 (3) | C24—H24B | 0.9900 |
| C9—C14 | 1.552 (4) | C25—C30 | 1.389 (4) |
| C10—C11 | 1.535 (4) | C25—C26 | 1.401 (4) |
| C10—H10A | 0.9900 | C26—C27 | 1.378 (4) |
| C10—H10B | 0.9900 | C26—H26 | 0.9500 |
| C11—C15 | 1.530 (4) | C27—C28 | 1.389 (5) |
| C11—C12 | 1.530 (4) | C27—H27 | 0.9500 |
| C11—H11 | 1.0000 | C28—C29 | 1.362 (4) |
| C12—C13 | 1.533 (4) | C28—H28 | 0.9500 |
| C12—H12A | 0.9900 | C29—C30 | 1.393 (4) |
| C12—H12B | 0.9900 | C29—H29 | 0.9500 |
| C13—C14 | 1.536 (4) | C30—H30 | 0.9500 |
| C13—C17 | 1.541 (5) | ||
| C1—N1—C2 | 108.75 (19) | C11—C15—H15B | 109.7 |
| C1—N1—C3 | 122.2 (2) | C16—C15—H15B | 109.7 |
| C2—N1—C3 | 128.9 (2) | H15A—C15—H15B | 108.2 |
| C2—N2—N3 | 104.9 (2) | C17—C16—C18 | 109.5 (2) |
| C1—N3—N2 | 113.09 (19) | C17—C16—C15 | 110.0 (3) |
| C1—N3—C19 | 126.6 (2) | C18—C16—C15 | 108.9 (2) |
| N2—N3—C19 | 119.69 (19) | C17—C16—H16 | 109.5 |
| C19—N4—C20 | 115.21 (19) | C18—C16—H16 | 109.5 |
| C19—N4—C23 | 114.27 (19) | C15—C16—H16 | 109.5 |
| C20—N4—C23 | 110.6 (2) | C16—C17—C13 | 109.4 (2) |
| C22—N5—C21 | 109.4 (2) | C16—C17—H17A | 109.8 |
| C22—N5—C24 | 111.6 (2) | C13—C17—H17A | 109.8 |
| C21—N5—C24 | 111.13 (19) | C16—C17—H17B | 109.8 |
| N3—C1—N1 | 103.3 (2) | C13—C17—H17B | 109.8 |
| N3—C1—S1 | 129.07 (19) | H17A—C17—H17B | 108.2 |
| N1—C1—S1 | 127.64 (18) | C16—C18—C9 | 110.6 (2) |
| N2—C2—N1 | 109.9 (2) | C16—C18—H18A | 109.5 |
| N2—C2—C9 | 122.1 (2) | C9—C18—H18A | 109.5 |
| N1—C2—C9 | 127.8 (2) | C16—C18—H18B | 109.5 |
| C4—C3—C8 | 121.4 (2) | C9—C18—H18B | 109.5 |
| C4—C3—N1 | 119.6 (2) | H18A—C18—H18B | 108.1 |
| C8—C3—N1 | 119.0 (2) | N4—C19—N3 | 116.7 (2) |
| C3—C4—C5 | 119.0 (2) | N4—C19—H19A | 108.1 |
| C3—C4—H4 | 120.5 | N3—C19—H19A | 108.1 |
| C5—C4—H4 | 120.5 | N4—C19—H19B | 108.1 |
| C4—C5—C6 | 119.9 (2) | N3—C19—H19B | 108.1 |
| C4—C5—H5 | 120.1 | H19A—C19—H19B | 107.3 |
| C6—C5—H5 | 120.1 | N4—C20—C21 | 108.8 (2) |
| C7—C6—C5 | 120.5 (2) | N4—C20—H20A | 109.9 |
| C7—C6—H6 | 119.8 | C21—C20—H20A | 109.9 |
| C5—C6—H6 | 119.8 | N4—C20—H20B | 109.9 |
| C8—C7—C6 | 120.0 (2) | C21—C20—H20B | 109.9 |
| C8—C7—H7 | 120.0 | H20A—C20—H20B | 108.3 |
| C6—C7—H7 | 120.0 | N5—C21—C20 | 109.7 (2) |
| C7—C8—C3 | 119.1 (2) | N5—C21—H21A | 109.7 |
| C7—C8—H8 | 120.4 | C20—C21—H21A | 109.7 |
| C3—C8—H8 | 120.4 | N5—C21—H21B | 109.7 |
| C2—C9—C18 | 108.7 (2) | C20—C21—H21B | 109.7 |
| C2—C9—C10 | 113.9 (2) | H21A—C21—H21B | 108.2 |
| C18—C9—C10 | 109.4 (2) | N5—C22—C23 | 109.6 (2) |
| C2—C9—C14 | 109.0 (2) | N5—C22—H22A | 109.7 |
| C18—C9—C14 | 107.8 (2) | C23—C22—H22A | 109.7 |
| C10—C9—C14 | 107.9 (2) | N5—C22—H22B | 109.7 |
| C11—C10—C9 | 110.3 (2) | C23—C22—H22B | 109.7 |
| C11—C10—H10A | 109.6 | H22A—C22—H22B | 108.2 |
| C9—C10—H10A | 109.6 | N4—C23—C22 | 109.52 (19) |
| C11—C10—H10B | 109.6 | N4—C23—H23A | 109.8 |
| C9—C10—H10B | 109.6 | C22—C23—H23A | 109.8 |
| H10A—C10—H10B | 108.1 | N4—C23—H23B | 109.8 |
| C15—C11—C10 | 109.1 (2) | C22—C23—H23B | 109.8 |
| C15—C11—C12 | 110.1 (2) | H23A—C23—H23B | 108.2 |
| C10—C11—C12 | 109.6 (2) | N5—C24—C25 | 113.1 (2) |
| C15—C11—H11 | 109.3 | N5—C24—H24A | 109.0 |
| C10—C11—H11 | 109.3 | C25—C24—H24A | 109.0 |
| C12—C11—H11 | 109.3 | N5—C24—H24B | 109.0 |
| C13—C12—C11 | 109.3 (3) | C25—C24—H24B | 109.0 |
| C13—C12—H12A | 109.8 | H24A—C24—H24B | 107.8 |
| C11—C12—H12A | 109.8 | C30—C25—C26 | 117.8 (3) |
| C13—C12—H12B | 109.8 | C30—C25—C24 | 121.9 (2) |
| C11—C12—H12B | 109.8 | C26—C25—C24 | 120.1 (3) |
| H12A—C12—H12B | 108.3 | C27—C26—C25 | 120.6 (3) |
| C12—C13—C14 | 109.9 (2) | C27—C26—H26 | 119.7 |
| C12—C13—C17 | 109.1 (3) | C25—C26—H26 | 119.7 |
| C14—C13—C17 | 109.4 (3) | C26—C27—C28 | 120.8 (3) |
| C12—C13—H13 | 109.5 | C26—C27—H27 | 119.6 |
| C14—C13—H13 | 109.5 | C28—C27—H27 | 119.6 |
| C17—C13—H13 | 109.5 | C29—C28—C27 | 119.2 (3) |
| C13—C14—C9 | 110.4 (2) | C29—C28—H28 | 120.4 |
| C13—C14—H14A | 109.6 | C27—C28—H28 | 120.4 |
| C9—C14—H14A | 109.6 | C28—C29—C30 | 120.6 (3) |
| C13—C14—H14B | 109.6 | C28—C29—H29 | 119.7 |
| C9—C14—H14B | 109.6 | C30—C29—H29 | 119.7 |
| H14A—C14—H14B | 108.1 | C25—C30—C29 | 121.0 (3) |
| C11—C15—C16 | 109.7 (2) | C25—C30—H30 | 119.5 |
| C11—C15—H15A | 109.7 | C29—C30—H30 | 119.5 |
| C16—C15—H15A | 109.7 | ||
| C2—N2—N3—C1 | 0.1 (3) | C17—C13—C14—C9 | −60.1 (3) |
| C2—N2—N3—C19 | 171.5 (2) | C2—C9—C14—C13 | 177.1 (2) |
| N2—N3—C1—N1 | 0.2 (3) | C18—C9—C14—C13 | 59.3 (3) |
| C19—N3—C1—N1 | −170.6 (2) | C10—C9—C14—C13 | −58.8 (3) |
| N2—N3—C1—S1 | 179.86 (19) | C10—C11—C15—C16 | −61.5 (3) |
| C19—N3—C1—S1 | 9.1 (4) | C12—C11—C15—C16 | 58.9 (3) |
| C2—N1—C1—N3 | −0.3 (3) | C11—C15—C16—C17 | −58.7 (3) |
| C3—N1—C1—N3 | 175.7 (2) | C11—C15—C16—C18 | 61.2 (3) |
| C2—N1—C1—S1 | 179.95 (19) | C18—C16—C17—C13 | −59.9 (3) |
| C3—N1—C1—S1 | −4.0 (4) | C15—C16—C17—C13 | 59.6 (3) |
| N3—N2—C2—N1 | −0.3 (3) | C12—C13—C17—C16 | −60.5 (3) |
| N3—N2—C2—C9 | −176.2 (2) | C14—C13—C17—C16 | 59.8 (3) |
| C1—N1—C2—N2 | 0.4 (3) | C17—C16—C18—C9 | 60.8 (3) |
| C3—N1—C2—N2 | −175.2 (2) | C15—C16—C18—C9 | −59.4 (3) |
| C1—N1—C2—C9 | 176.0 (2) | C2—C9—C18—C16 | −177.5 (2) |
| C3—N1—C2—C9 | 0.3 (4) | C10—C9—C18—C16 | 57.6 (3) |
| C1—N1—C3—C4 | 83.1 (3) | C14—C9—C18—C16 | −59.5 (3) |
| C2—N1—C3—C4 | −101.8 (3) | C20—N4—C19—N3 | −65.8 (3) |
| C1—N1—C3—C8 | −97.5 (3) | C23—N4—C19—N3 | 63.8 (3) |
| C2—N1—C3—C8 | 77.6 (3) | C1—N3—C19—N4 | −103.1 (3) |
| C8—C3—C4—C5 | −2.9 (4) | N2—N3—C19—N4 | 86.8 (3) |
| N1—C3—C4—C5 | 176.5 (2) | C19—N4—C20—C21 | −169.2 (2) |
| C3—C4—C5—C6 | 0.5 (4) | C23—N4—C20—C21 | 59.4 (3) |
| C4—C5—C6—C7 | 1.5 (4) | C22—N5—C21—C20 | 60.9 (3) |
| C5—C6—C7—C8 | −1.0 (4) | C24—N5—C21—C20 | −175.4 (2) |
| C6—C7—C8—C3 | −1.3 (4) | N4—C20—C21—N5 | −60.1 (3) |
| C4—C3—C8—C7 | 3.3 (4) | C21—N5—C22—C23 | −60.0 (3) |
| N1—C3—C8—C7 | −176.1 (2) | C24—N5—C22—C23 | 176.6 (2) |
| N2—C2—C9—C18 | 88.9 (3) | C19—N4—C23—C22 | 169.1 (2) |
| N1—C2—C9—C18 | −86.1 (3) | C20—N4—C23—C22 | −59.0 (3) |
| N2—C2—C9—C10 | −148.8 (2) | N5—C22—C23—N4 | 58.8 (3) |
| N1—C2—C9—C10 | 36.1 (4) | C22—N5—C24—C25 | −75.8 (3) |
| N2—C2—C9—C14 | −28.3 (3) | C21—N5—C24—C25 | 161.8 (2) |
| N1—C2—C9—C14 | 156.6 (2) | N5—C24—C25—C30 | −14.8 (4) |
| C2—C9—C10—C11 | −179.4 (2) | N5—C24—C25—C26 | 169.7 (2) |
| C18—C9—C10—C11 | −57.6 (3) | C30—C25—C26—C27 | −0.2 (4) |
| C14—C9—C10—C11 | 59.4 (3) | C24—C25—C26—C27 | 175.5 (3) |
| C9—C10—C11—C15 | 59.7 (3) | C25—C26—C27—C28 | 0.5 (4) |
| C9—C10—C11—C12 | −61.0 (3) | C26—C27—C28—C29 | −0.4 (5) |
| C15—C11—C12—C13 | −60.0 (3) | C27—C28—C29—C30 | 0.1 (4) |
| C10—C11—C12—C13 | 60.1 (3) | C26—C25—C30—C29 | −0.1 (4) |
| C11—C12—C13—C14 | −59.5 (3) | C24—C25—C30—C29 | −175.7 (3) |
| C11—C12—C13—C17 | 60.5 (3) | C28—C29—C30—C25 | 0.2 (4) |
| C12—C13—C14—C9 | 59.7 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C13—H13···S1i | 1.00 | 2.85 | 3.751 (3) | 150 |
| C28—H28···S1ii | 0.95 | 2.84 | 3.673 (4) | 146 |
Symmetry codes: (i) x+1, y, z; (ii) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5419).
References
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- El-Emam, A. A. & Ibrahim, T. M. (1991). Arzneim. Forsch./Drug Res. 41, 1260–1264. [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681105570X/xu5419sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681105570X/xu5419Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681105570X/xu5419Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report