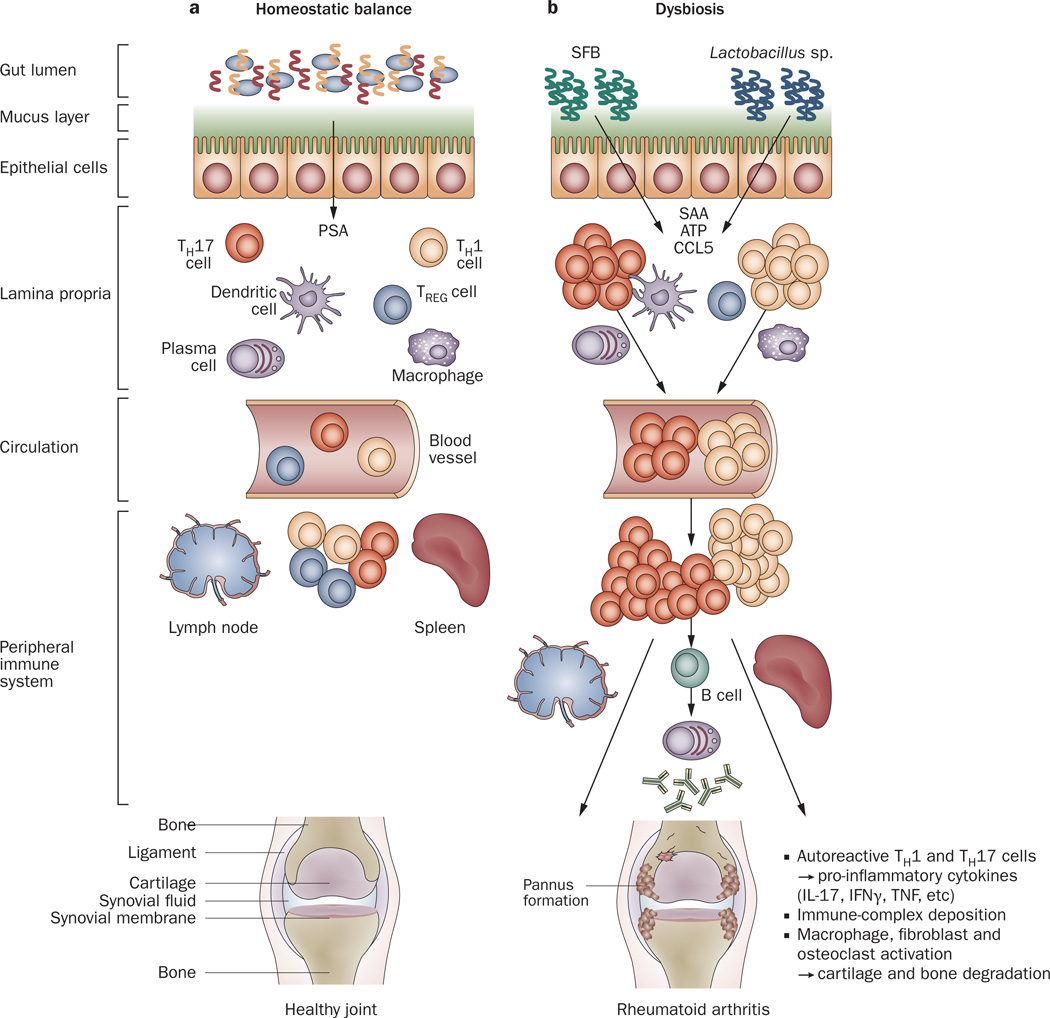
Figure 3.
Host–microbiota interactions in health and inflammatory arthritis. a | In healthy individuals, a well balanced host–microbial cross-talk is essential for the maintenance of homeostasis. A thick mucus layer and epithelial cells prevent direct contact with the gut-associated immune cells, which constantly survey the contents of the intestinal lumen and eliminate undesired antigens. Commensal bacteria, such as Bacteroides fragilis, can activate pro-tolerogenic machinery. A specific cell wall component, PSA, is sufficient to induce TREG-cell activation, IL-10 production and TH17-cell repression to avoid uncontrolled inflammation. b | When either genetic or environmental factors alter the balance in the microbiota composition, dysbiosis ensues. Potentially harmful micro-organisms (such as SFB or Lactobacillus) predominate and local expansion of proinflammatory cells (TH17 cells, TH1 cells and others) occurs via different molecules (such as ATP, SAA or CCL5 signaling). These autoreactive T cells migrate to peripheral immune compartments and activate B cells to differentiate into autoantibody-producing plasma cells. These cells and antibodies then migrate to synovial tissue where the inflammatory cascade is amplified through the activation of effector components, including macrophages, fibroblasts, osteoclasts, cytokines and proteinases. If self-perpetuating, this process can lead to arthritis and pannus formation. Abbreviations: ATP, adenosine-5'-triphosphate; CCL5, CC-chemokine ligand 5; IFNγ, interferon γ; IL-17, interleukin-17; PSA, polysaccharide A; SAA, serum amyloid A; SFB, segmented filamentous bacteria; TH1, type 1 T helper cell; TH17, type 17 T helper cell; TREG, regulatory T cell.