Abstract
Introduction
The intraoperative localization of suspicious lesions detected by positron emission tomography (PET) scan remains a challenge. To solve this, two novel probes have been created to accurately detect the 18F-FDG radiotracer intraoperatively.
Methods
Nude rats were inoculated with mesothelioma. When PET scans detected 10-mm tumors, animals were dissected and the PET probes analyzed the intraoperative radiotracer uptake of these lesions as tumor to background ratio (TBR).
Results
The 17 suspicious lesions seen on PET scan were localized intraoperatively (by their high TBR) using the PET probes and found malignant on pathology. Interestingly, smaller tumors not visualized on PET scan were detected intraoperatively by their high TBR and found malignant on pathology. Furthermore, using a TBR threshold as low as 2.0, both gamma (sensitivity, 100%; specificity, 80%; positive predictive value (PPV), 96%; and negative predictive value (NPV), 100%) and beta (sensitivity, 100%; specificity, 60%; PPV, 93%; and NPV, 100%) probes reliably detected suspicious lesions on PET scan imaging. They also showed an excellent area under the curve of 0.9 and 0.97 (95% CI of 0.81–0.99 and 0.93–1.0) for gamma and beta probes, respectively, in the receiver operating characteristic analysis for detecting malignancy.
Conclusion
This novel tool could be used synergistically with a PET scan imaging to maximize tissue selection intraoperatively.
Keywords: PET probe, Minimally invasive surgery, Intraoperative probe, Beta rays, High-energy gamma rays
Introduction
The ability to detect tumor deposits is one of the most desired goals of the oncological field. Multiple imaging modalities have been developed and tested to solve this task in a non-interventional manner. These tests have had significant improvements over the last few decades and as a result, the finding of a suspicious lesion requiring further workup is increasingly seen in the medical field. Once such a lesion is found, its intraoperative localization becomes crucial for its proper diagnosis and treatment. It is at this stage that surgeons become involved to obtain a reliable biopsy of the lesion of interest. And even though, this is probably the most important step for its diagnosis, biopsies are mostly obtained with nonspecific tumor tools such as needle localization, tissue palpation or direct intraoperative visualization. Thus, increasing advances in the radiological detection of malignancies have not yet seen parallel improvements in the surgical field. Therefore, the intraoperative localization of these suspicious lesions detected on imaging provides the rate limiting step in today’s diagnostic workup equation.
The positron emission tomography (PET) scanning is one of the most commonly used imaging modalities by which cancer and recurrences are being diagnosed and monitored. This tomography detects different radiotracers attached to specific molecules of interest. In recent years, there have been major advances in radiotracer selection and usage.1, 2 But although PET scanners have improved tumor detection, this test is currently not well suited for intraoperative use because of limitations in availability of intraoperative units, cost, and the cumbersome nature of such device in the operative environment. Furthermore, this tomographic test cannot delineate the precisely tri-dimensional locations of the tissues of interest.3
In response to these drawbacks, there has been a parallel development of novel molecular-guided technology in the form of handheld probes for use in the operating room. These devices are designed to detect either low- or high-energy particles emitted from previously selected radiotracers. As an example of their applications, the use of low-energy gamma probes to detect technetium is standard for lymphoscintigraphy and broadly used today in interventional procedures.
Other radiotracers have imposed greater challenges for their intraoperative detection, including the F-18-labeled fluorodeoxyglucose (18F-FDG), which is the most currently used radioisotope for PET scan imaging. This compound has shown a high sensitivity and specificity for detection of malignant tissue, but the challenges for its use during operative procedures are due to its intrinsic characteristics. This isotope emits two rays, a low-energy beta ray and a high-energy gamma ray. It is the high-energy gamma particle which creates a significant surrounding interference and therefore substantial problems on its precise localization intraoperatively. Nonetheless, two novel intraoperative probes have been created to solve this issue and reliably detect this radioisotope. These probes are a handheld device and each can detect either of the two rays emitted by this radiotracer: gamma and beta. The 18F-FDG emitted gamma radiation (the high-energy particle) travels a long distance and can even be detected outside of the body cavity. It is this characteristic that makes it appealing for the PET scan imaging. But for the intraoperative probe to reliably detect these gamma rays and avoid its surrounding radiation, it has to have a large amount of heavy shielding, and is therefore large and bulky. On the other hand, the beta rays only travel millimeters before annihilating into two high-energy gamma rays.4 Therefore, this second rays can only be detected intraoperatively, and are affected to a much less degree by its surrounding radiation. In fact, the main advantage of the beta probe (the probe designed to detects the beta rays) is that it does not need the high degree of collimation needed on the gamma probe (the probe designed to detect the gamma rays), and is therefore smaller in diameter and more appealing for its intraoperative usage, especially for minimally invasive procedures.
The intraoperative use of this device is simple. It detects the amount of radiation emitted by a specific source, and gives the result as a number, which units are counts per seconds. This number is then easily standardized to the patient’s background uptake and is interpreted as tumor to background ratio (TBR). As a result, this ratio is directly proportional to the amount of radiotracer uptake from that specific location and subsequently to its malignant potential. Thus, the higher the TBR, the more likely the lesion is malignant.
We designed a study to assess the ability of this intraoperative device to localize suspicious tissue previously seen on PET scan. Our first aim is to correlate the PET scan imaging findings with that of the intraoperative gamma and beta probes.
Our second aim is to define the limits of detection of the radiologic PET scan exam, as it is generally accepted that smaller tumors are not well detected using this test.5 Similarly, we evaluated the limits of detection of these intraoperative probes and correlated tumor sizes with their radiotracer uptake.
Overall, our aims focus on defining the usefulness of these probes for the intraoperative guidance and selection of specific lesions when complemented with a preoperative PET scan imaging, taking into account different tumor sizes.
Materials and Methods
Animal Care
Nude rats described (averaged 250 g), all comply with the regulatory requirements of the Institutional Animal Care and Use Committee, the Research Animal Resource Center (RARC) of MSKCC, and the National Institutes of Health (NIH) “Guide for the Care and Use of Laboratory Animals”.
They were all fed ad libitum, and maintained on broad spectrum prophylactic antibiotics upon their arrival to the institution. All animal procedures were performed under proper inhaled anesthesia using 2% isoflurane. Animals were killed via CO2 inhalation just prior to necropsy.
Cell Line
The human mesothelioma line was selected for its xenograph uptake quality in nude rats previously reproduced in our laboratory (unpublished data). This line was maintained with Roswell Park Memorial Institute Medium+10% FCS P+S+10 mM HEPES+2 mM fl-glutamine+1 mM sodium pyruvate and 1.5 g/L sodium bicarbonate+4.5 g/l-glucose. Cells were kept in a 5% CO2 humidified incubator at 37°C and subcultured twice weekly.
Irradiation
Nude rats although athymic, are immunocompetent to a certain degree and therefore resist a large variety of implanted tumors. To increase the uptake of our xenograft model, their immune function was further suppressed by external beam radiation.6–8 Using the Gammacel 40 whole-body radiator, the animals received a one-time dose of 500 cGy. Desirable immunosuppression is obtained 4 days after this procedure, and xenograft implantation was performed then. Radiation produces no pain or discomfort to animals, and therefore no anesthesia was required.
For optimal preparation of this immunosuppression, animals were treated with prophylactic antibiotics upon their arrival to our institution. Antibiotic treatment was continued after whole-body radiation to avoid secondary, opportunistic infections.
Xenograft Implantation
After desired immunosuppression of the animals was achieved, 2e-7 cultured cells in 100 µl PBS suspension were intrapleurally injected over the right and left side of their chest wall. For this procedure, a 1 cm skin incision was performed in each site, with the animals under inhaled isoflurane anesthesia. The dissection was continued until the rib cage was visualized. Then, the syringe containing the suspended cells was carefully introduced intrapleurally, and the suspended cells injected. Each wound was closed using surgical clips, which were subsequently removed several days after the procedure. All animals tolerated the procedure well and were closely monitored with weekly nuclear imaging studies.
Radioisotope Production and Injection into Rats
18F-FDG was obtained from the institutional radiopharmacy laboratory (Nuclear Medicine Department, MSKCC, New York, NY). A total volume of 0.15–0.20 ml containing 500 µCi of 18F-FDG in sterile PBS was injected retro-orbitally in each rat under inhaled, isoflurane anesthesia.
MicroPET Scan Imaging
Animals were starved overnight to enhance the radiotracer uptake. After retro-orbital injection of 500 µCi of 18F-FDG, a 1-h period was allowed for optimal radiotracer uptake,9 and then the animals were placed in a prone position on the scanner.
Scans were performed with transaxial fields of view of 10 cm and s axial views using the Focus 120 micro-PET™ dedicated small-animal PET scanners (Concorde Microsystems, Knoxville, TN). The transaxial field of view covered from the lower neck to the upper abdomen. Scans were collected with an energy window of 350–750 KeV and a coincidence timing window of 6 ns. Data was sorted into 2D histograms by Fourier re-binning and transverse images were reconstructed in a 128 × 128 × 63 (R4) or 128 × 128 × 96 (Focus 120) matrices by filtered back-projection. Images were corrected for non-uniformity of scanner response, and radionuclide decay to the time of injection.
Evidence of high 18F-FDG uptake lesions was found 2 to 4 weeks after tumor implantation. Those lesions were followed until desired tumor size was reached (10 mm), and dissection with probe readings was performed then.
Procedure
Dissection
Once suspicious lesions where readily visualized on PET scan imaging, the animals were brought to the operating table. A retro-orbital injection of 500 µCi 18F-FDG was performed and animals were then kept under proper inhaled anesthesia for a total of 30 min. Dissection was then performed, so that tissue sampling could take place 60–90 min after the injection for optimal radioisotope absorbance (as explained above). Animals were then killed for dissection. During the procedure, particular interest was given to the high 18F-FDG uptake areas previously visualized on PET scan. The hand-held probe was placed perpendicularly over those areas and dissection was carried with the guidance of the high radioisotope counts obtained by the probes. Once the mass was directly visualized, in situ readings were recorded in triplicates. Afterwards, these lesions were dissected off the surrounding tissue and fresh frozen for subsequent pathological analysis.
Pet Probe and Counts
The high-energy gamma and beta probes (IntraMedical Imaging LLC, Los Angeles, CA) are designed to detect 511-keV photons from positron-emitting sources (gamma probe) and positrons (or beta rays) directly (beta probe). The probes were calibrated to accurately localize the point source of 18F-FDG and the count rate was determined to optimize the detection of the 511 keV emissions.
Tumors were kept in situ for analysis. Radioactive emissions were measured in counts per second and recorded in triplicate for both beta and gamma probes.
A background tissue was obtained to correct for the background uptake of this radiotracer. The psoas muscle was selected as it is easily located inside the body cavity, and also is in close proximity to surrounding high FDG uptake organs, making it similarly affected to the surrounding radiation as the tumors to be biopsied. Readings were performed in triplicates in each animal, for TBR calculations.
Image Analysis
PET image analysis was done with ASIPro™ software (Concorde Microsystems Inc., Knoxville, TN). To verify ROI measurements, selected tissues were harvested, weighed, and counted in triplicate in a scintillation well counter calibrated for 18F-FDG.
Statistical Analysis
Receiver operating characteristic (ROC) curves were utilized to assess the ability of each probe to detect PET-positive lesions as well as malignant tissue, and the area under the ROC curve (AUC) was used to summarize these measurements. Each ROC curve and its AUC was estimated non-parametrically and an optimal threshold was identified using the maximal Youden index.10 A simple way to analyze the ROC graphs is the closer the AUC is to 1.0, in other words the closer the curves are to the left upper quadrant of the graph, the better the overall performance of the probes.
Other methods of finding an optimal threshold, such as the maximal chi-square method were not considered since their power would have been limited in this relatively small sample.
Results
After 2 to 4 weeks of monitoring, tumors reached desirable sizes (10mm) in PET scan studies (Fig. 1). They were mostly located on the chest wall of the animals as well as in the intrapleural space. Some intrapleural tumors grew aggressively, and no anatomical distinction could be made between thoracic structures and tumor masses. On the other hand, other tumors as well as tumors on the chest wall were well delineated and amenable for precise surgical resection and probe analysis.
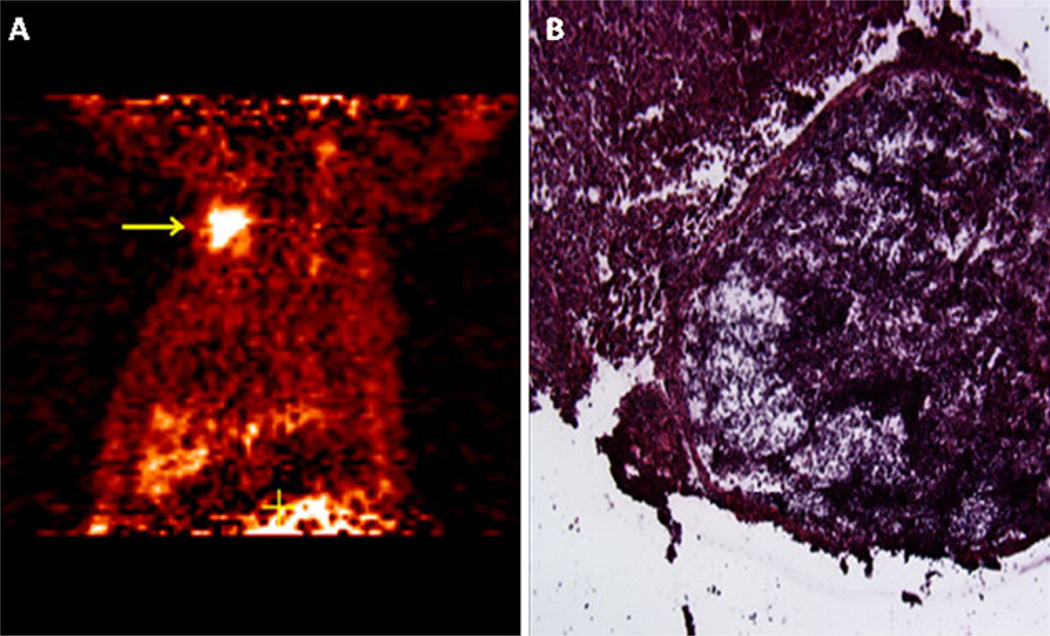
Fig. 1.
PET scan imagine and pathological results. a Suspicious right chest wall mass. The high radiotracer uptake seen in this PET scan study correlated with the high TBR detected with the intraoperative probes. TBR of 11.0 and 8.24 were detected for gamma and beta probes, respectively. b H&E staining of the lesion shows mesothelioma
A total of 17 suspicious masses were found on PET scan imaging (named PET-scan-positive tumors), and all were subsequently localized intraoperatively with the use of either probe. Localization of tumors was sometimes facilitated by the evident tumor mass, but other times (on smaller tumors) by intraoperative explorations using the probes to guide the curse of the dissection. Probe readings, along with size measurements of the tumors were performed in situ, followed by tissue dissection and pathological analysis. Subsequently, all of these 17 lesions were found malignant on pathological examination.
Intraoperatively, all PET-scan-positive tumors were noted to have a higher TBR compared with their surrounding tissues. For the gamma probe, the average TBR value was 7.7, ranging from 4.4 to 23.4. The beta probe had a higher average of 8.7, ranging from 3.2 to 19. For comparison purposes, surrounding benign chest wall tissue TBRs was also calculated for both probes. The gamma probe had an average of 1.1, and a range from 0.72 to 1.6. Similar values for the beta probe averaged 1.4, ranging from 0.66 to 2.2. Benign pleural and peritoneal tissue samples produced similar results. As seen, both probes were able to locate all suspicious tumors previously seen on PET scan, with a wide difference between their TBR and that of the other surrounding, benign tissue. In fact, using a TBR as low as 2.5, both probes reliably located all suspicious tissues previously seen on imaging.
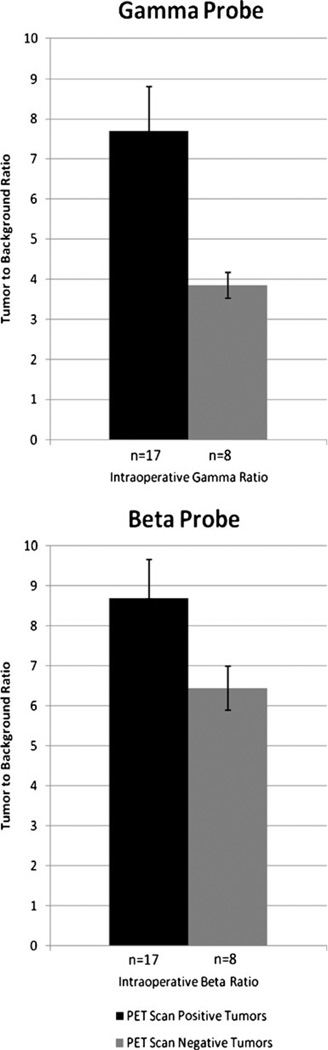
Interestingly, we also encountered other small tumors with high intraoperative TBR, but not detected on the PET scan studies. A total 8 of these lesions were found, and ultimately proved malignant on pathological examination. These were defined as PET-scan-negative tumors. Their size and probe counts were analyzed in situ and recorded again in triplicate. TBRs on these malignant lesions were higher than that of their surrounding benign tissue, but somewhat lower than the previously described PET-scan-positive tumor group. Values on the gamma probe ranged from 2.4 to 5.6, with an average of 3.9. For the beta probe, values ranged from 3.1 to 7.8, for an average value of 6.44. Figure 2 compares the intraoperative TBR between the PET-scan-positive and PET-scan-negative tumors. Of note, the beta probe had a smaller difference between these two groups.
Fig. 2.
Gamma and beta TBR of positive versus negative tumors on PET scan. Both probes detected an increased uptake on the PET-scan-positive group. SEM of both probes are shown for comparison. n number of tumors sampled, SEM standard error of the mean
The overall ability of the intraoperative probes to detect malignant tissue regardless of the PET scan results or tumor diameters is shown on Table 1. Increasing the TBR from 1.5 correlated to an increased ability of both probes to differentiate between malignant and benign tissue. From a TBR of 2.0 to 3.0, both probes had their optimal results, suggesting that the cut off value for tissue sampling could be found in this range.
Table 1.
Intraoperative probes detecting malignant tissue
| Probes localizing malignancy (n=25) | ||||||||
|---|---|---|---|---|---|---|---|---|
| TBR | ≥1.5 | ≥2.0 | ≥2.5 | ≥3.0 | ||||
| Gamma probe | Sensitivity | 100.0 | Sensitivity | 100.0 | Sensitivity | 95.8 | Sensitivity | 91.7 |
| Specificity | 80.0 | Specificity | 80.0 | Specificity | 100.0 | Specificity | 100.0 | |
| PPV | 96.2 | PPV | 96.0 | PPV | 100.0 | PPV | 100.0 | |
| NPV | 100.0 | NPV | 100.0 | NPV | 83.3 | NPV | 71.4 | |
| Beta probe | Sensitivity | 100.0 | Sensitivity | 100.0 | Sensitivity | 100.0 | Sensitivity | 100.0 |
| Specificity | 60.0 | Specificity | 60.0 | Specificity | 100.0 | Specificity | 100.0 | |
| PPV | 92.6 | PPV | 92.6 | PPV | 100.0 | PPV | 100.0 | |
| NPV | 100.0 | NPV | 100.0 | NPV | 100.0 | NPV | 100.0 | |
This table demonstrates the overall ability of the probes to detect malignant tissue regardless of their sizes. As shown, both probes had excellent sensitivities between TBR of 2.5 and 3.0. The beta probe had a more definite cut off value of 2.5 than the gamma probe
PPV positive predictive value, NPV negative predictive value, n number of samples
Of note, tumors detected on PET scan had larger diameters, with an average of 14 mm. These were significantly larger than the 8-mm average diameter found on the PET-scan-negative group. Interestingly, there is some overlapping between the two groups, especially between 9.0 and 11.9 mm. Below and above this diameter, the PET scan test was consistent in either not detecting them at all (if less than 9 mm), or reliably detecting them (if larger than 11.9 mm).
This finding is consistent with previous studies which show that tumors less than 1 cm in diameter are not accurately detected in PET scan imaging.5 In our study group, we divided the tumors by size to better assess and compare the limits of detection of both the PET scan and the intraoperative PET probes. Table 2 shows the statistical results for the PET scan imaging for detecting tumor masses either larger than or smaller than 1 cm in diameter. For those over 1 cm, the PET scan had a sensitivity of 87% and a specificity of 100%. These results were significantly altered for tumors less than 1 cm in diameter, as the sensitivity of this imaging exam dropped to 40%.
Table 2.
PET scan results for tumor detection
| PET scan results for tumor detection | |||
|---|---|---|---|
| Tumor size | <1 cm | ≥1 cm | All tumors |
| Sensitivity | 40 | 87 | 68 |
| Specificity | 100 | 100 | 100 |
| PPV | 100 | 100 | 100 |
| NPV | 80 | 92 | 76 |
This table shows the ability of the PET scan to detect tumors depending on their sizes. A significant decrease in sensitivity is observed on tumors less than 1 cm in diameter
PPV positive predictive value, NPV negative predictive value, n number of samples
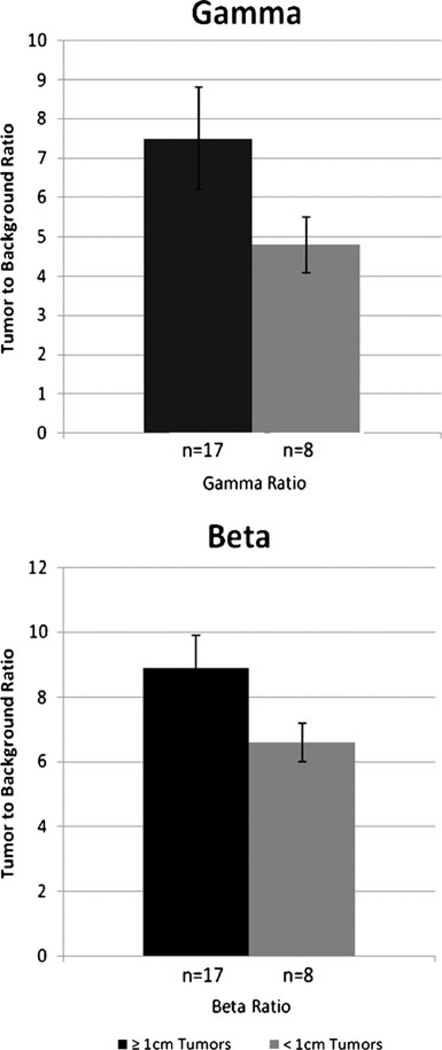
On the other hand, the intraoperative probes showed better results for detecting tumors smaller than 1 cm. Figure 3 compares the corresponding TBRs of these two groups. For tumors less than 1 cm in diameter, the gamma probe had an average TBR of 4.8 (ranging from 2.6 to 10.6) and that for the beta probe was 6.6 (ranging from 3.2 to 9.0). Using a cutoff TBR of 1.5, both probes had a sensitivity and NPV of 100% for detecting these smaller sized tumors, with somewhat lower specificity for the beta probe (60% vs 80%). For gamma and beta probes, their corresponding PPV were 91 and 83, respectively.
Fig. 3.
Gamma and beta TBR of tumors larger versus smaller than 1 cm in diameter. Higher values are noted over the larger tumor group for both probes. A smaller difference between the groups is noted on the beta probe. This underscores the improved limits of detection that could be obtained by this probe. SEM of both probes are shown for comparison. n number of tumor sample, SEM standard error of the mean
For those larger than 1 cm in diameter, average TBR values for both gamma and beta probes were 7.5 (ranging from 3.3 to 23) and 8.9 (ranging from 3.6 to 19), respectively. Here, both probes had excellent results using a cutoff TBR of 2.5. In fact, for a TBR of 1.5, both probes had sensitivity and NPV of 100%. And similar to the results for the smaller tumors, the specificity of the gamma probe was somewhat higher (80% vs 60%), as well as the PPV (93% vs 88%).
But even though using this small TBR showed some benefit for the gamma probe, using the ROC curves, the overall consistency of TBRs in malignant tissues regardless of the tumor sizes showed better results for the beta probe (AUC of 0.90 and 0.97; 95% CI of 0.81–0.99 and 0.93–1.0 for gamma and beta probes, respectively).
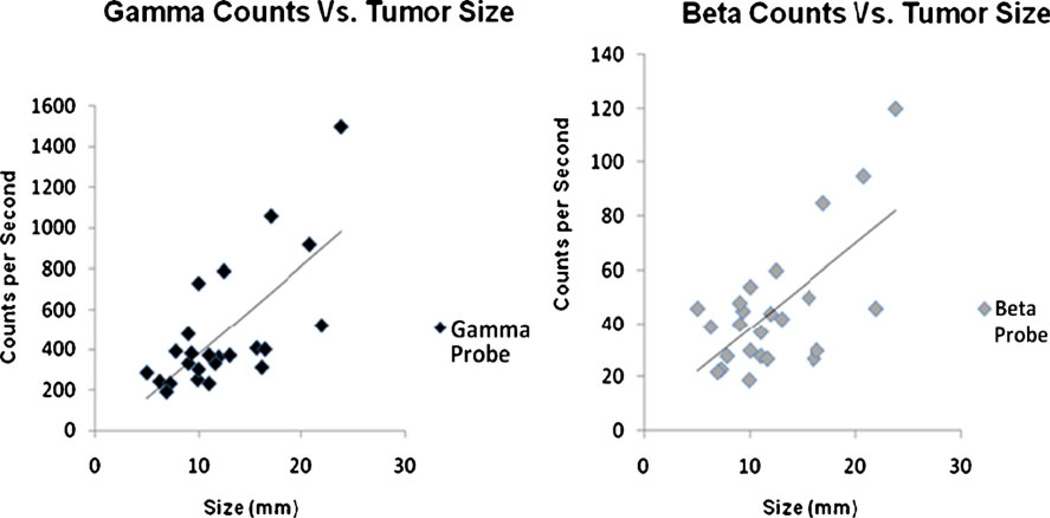
Finally, we were able to correlate tumor diameters with intraoperative counts detected on both probes. Figure 4 demonstrates the positive correlation between size and counts per second detected, with an R2 value of 0.48 and 0.43 for both gamma and beta probes, respectively. This again shows the ability of the probes to detect the higher 18F-FDG uptake expected on larger tumor masses.
Fig. 4.
Gamma and beta probe counts versus tumor size. A comparison between intraoperative counts obtained by the probes and the respective tumor sizes. This count is directly proportional to the degree of isotopic radiation emitted by the tissue being measured. As described, this number is easily calibrated to TBR after dividing it by the background’s value. A direct correlation was seen, confirming the higher uptake detected on the larger sized tumors. Calculated R2 resulted in 0.48 and 0.43 for gamma and beta probes, respectively
Discussion
The proper and reliable intraoperative detection of suspicious lesions seen on PET scan is essential for the correct diagnosis and treatment of cancer patients. By providing an intraoperative tool to better localize these tissues, a more accurate classification and staging could be obtained, with a secondary advantage of avoiding unnecessary tissue resection to obtain a reliable sample in such procedures. Furthermore, it will add an element to guide surgeons when an intraoperative assessment significantly changes operative planning, as is known to occur up to one third of cases.11
As mentioned before, 18F-FDG is the most commonly used radioisotope in PET scan exams, and numerous studies have shown its high sensitivity and accuracy for evaluating both primary and metastatic diseases.12–22 This molecule is a fluorinated glucose analog, and is internalized in cells using the GLUT transporters. It is then selectively concentrated in tumor tissue,23 and from there emits two types of waves: gamma and beta. The first emitted wave (beta) is a positron that travels a short distance and eventually collides with a nearby electron to produce two gamma rays, 180° apart from each other.4 This subsequent wave emitted is a high-energy ray (511 KeV) that can travel several centimeters in tissue.
The hand held PET probe is an intraoperative devise first described and developed by Daghighian et al.24 in 1994, as a novel method to direct intraoperative tumor localization. These positron detecting devices allow for direct localization of radiolabeled tumor cells by detecting both, gamma and beta rays. The high degree of collimation required to efficiently detect gamma rays, substantially increases the diameter of this devise and impedes its ideal mechanical manipulation intraoperatively. This is accentuated on procedures in small body cavities, or during minimally invasive surgeries. In contrast, the beta probe does not need such protection. This is because it is made from a thin crystal, sufficient to stop electron radiation, but too thin to be affected by the surrounding gamma rays. It therefore has a smaller diameter and is ideal for intraoperative manipulations as well as for minimally invasive procedures, as it very well fits in a 5- mm port used for laparoscopic surgery. Furthermore, the shorter path traveled by these rays makes it ideal for tumor detection at a closer range, as well as localization of smaller tumor deposits, as it is less affected by surrounding radiation than the gamma probe. These facts, along with its smoother intraoperative handling, may allow for better identification of metastatic foci,24 and thus obviate unnecessary tissue resection in particular patients (such as those with pancreatic cancer and detectable peritoneal spread).
In this study, we focused on the importance of the intraoperative localization of tumors noted on PET scan imaging. As our results show, we were able to locate each of those suspicious lesions with either probe. Moreover, the direction of the dissection was guided on multiple occasions by the high counts detected by the probes towards the areas of interest.
PET scan imaging found 17 out of the total 25 tumors present. It failed to detect some tumors smaller than 1 cm in diameter. This is highlighted by the drop on its sensitivity from 87% to 40% when comparing tumors larger versus those smaller than one centimeter in diameter. On the other hand, the intraoperative probes were substantially better at detecting smaller tumors. The beta probe had consistently better results over the gamma probe in detecting tumors smaller than 1 cm in diameter, as shown in the ROC curves explained above. These suggest that improved limits of detection, as well as the possibility of analyzing the margins of a resected specimen could be attained using this novel, intraoperative tool.
Figure 2, shows a wider difference on the gamma probe between the two groups being compared. This shows a more direct correlation between the gamma probe and the PET scan findings. It may also disclose a somewhat better sensitivity for malignancy detection of the beta probe, particularly over the smaller tumor masses. In other words, the gamma probe may replicate better the results obtained by PET scan imagine, but the beta probe was somewhat more selective for malignant tissue, especially over the smaller tumors lesions. A proposed explanation of this finding is that the PET scanning was significantly affected by the size of the lesion. Below a certain threshold (in this case 9.0 mm) it was more likely to be unable to detect the malignant tissue. Similarly the gamma probe was less able to precisely locate these smaller tumors. On the other hand, the beta showed some advantages detecting smaller malignant lesions, possibly because it was affected to a lesser degree by its surrounding radiation.
Finally, the different tumor sizes (from 4.5 to 23.8 mm in diameter) were correlated with their intraoperative radioisotope emission obtained as counts per second. A direct, positive correlation was observed on both probes. This again shows the reproducibility of this intraoperative device for detecting different amounts of radiotracer uptake given by the various tumor sizes.
Based on our results, we suggest that the optimal TBR for localizing malignant lesions may be between 2.0 and 3.0 for both probes. That is about two times the isotopic radiation emitted from the background tissues. Within this range, the sensitivity of tissue selection was kept at its highest, and the specificity, as well as the PPV had the most substantial increase.
It is worth mentioning, that our comparison of the different probes was not intended to define one superior to the other. In fact, it is the synergism of the two what may be needed in most cases. While the gamma probe could easily guide you with certainty towards the PET scan findings, the beta probe might make possible the detection of smaller tumor foci, or an improved assessment of malignant tissues over an anatomically smaller areas. Furthermore, this later probe may guide surgeons to better select specific areas for tissue dissection, and even assess the margins of a resected specimen.
Conclusions
The intraoperative probes were able to localize suspicious lesions previously seen on PET scan. Each probe offers its own advantages and are thus designed to be easily interchanged during the surgical procedure. The reproducibility of these results over the multiple tumor samples demonstrates that this tool can be used with confidence in the operative environment. Furthermore, when this devise is complemented with a PET scan imagine, it exponentially improve our ability to efficiently localize malignancies during surgical procedures.
Contributor Information
Segundo Jaime González, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Lorena González, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Joyce Wong, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Peter Brader, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Maureen Zakowski, Department of Pathology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Mithat Gönen, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Farhad Daghighian, IntraMedical Imaging LLC, Los Angeles, CA, USA.
Yuman Fong, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA.
Vivian E. Strong, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA, strongv@mskcc.org
References
- 1.Andrei Iagaru, Andrew Quon. Advances in Metabolic Imaging for Surgical Oncology. Surg Oncol Clin N Am. 2007;16:273–292. doi: 10.1016/j.soc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Strong VE, Humm J, Russo P, Jungbluth A, Wong D, Daghighian F, Old L, Fong Y, Larson SM. A novel method to localize antibody-targeted cancer deposits intraoperatively using handheld PET beta and gamma probes. Surg Endosc. 2007 Nov;22(2):386–391. doi: 10.1007/s00464-007-9611-3. [DOI] [PubMed] [Google Scholar]
- 3.Foehrenbach H, Albérini JL, Maszelin P, Bonardel G, Tenenbaum F, de Dreuille O, Richard B, Gaillard JF, Devaux JY. Positron emission tomography in clinical oncology. Presse Med. 2003 Feb 15;32(6):276–283. [PubMed] [Google Scholar]
- 4.Gambhir Sanjiv Sam, et al. Molecular Imaging Of Cancer With Positron Emission Tomography. Nature Reviews, Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 5.Naresh C Gupta, et al. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest. 2000 March;117(no. 3):773–778. doi: 10.1378/chest.117.3.773. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon C, et al. Heterotransplantation of human cancer I. Irradiated rats. Cancer Res. 1952;12:909–911. [PubMed] [Google Scholar]
- 7.Howard RB, Chu H, Zeligman BE, et al. Irradiated nude rat model for orthotopic human lung cancers. Cancer Res. 1991;51 3274±80. [PubMed] [Google Scholar]
- 8.Kjonniksen I, Nesland JM, Pihl A, Fodstad O. Nude rat model for studying metastasis of human tumor cells to bone and bone mar row. J Nat Canc er Inst. 1990;82 doi: 10.1093/jnci/82.5.408. 408±12. [DOI] [PubMed] [Google Scholar]
- 9.Higashi T, Saga T, Ishimori T, Mamede M, Ishizu K, Fujita T, Mukai T, Sato S, Kato H, Yamaoka Y, Matsumoto K, Senda M, Konishi J. What is the most appropriate scan timing for intraoperative detection of malignancy using 18F-FDG-sensitive gamma probe? Preliminary phantom and preoperative patient study. Ann Nucl Med. 2004;18:105–114. doi: 10.1007/BF02985100. [DOI] [PubMed] [Google Scholar]
- 10.Gonen M. Analyzing receiver operating characteristic curves with SAS. Cary, NC: SAS Press; 2007. [Google Scholar]
- 11.Schwarz RE. Factors influencing change of preoperative treatment intent in a gastrointestinal cancer practice. World J Surg Oncol. 2007 Mar 13;5:32. doi: 10.1186/1477-7819-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton C, Ell P, Linch D. The role of PET imaging in lymphoma. Br J Haematol. 2004;126:772–784. doi: 10.1111/j.1365-2141.2004.05069.x. [DOI] [PubMed] [Google Scholar]
- 13.van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–3812. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 14.Gulec SA, Faries MB, Lee CC, Glass E, Morton DL, Essner R. The role of FDG-PET in the management of patients with metastatic melanoma: impact on surgical decision making. Clin Nucl Med. 2003;28:961–965. doi: 10.1097/01.rlu.0000099805.36471.aa. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Alavi A. PET imaging in gynecologic malignancies. Radiol Clin North Am. 2004;4:1155–1167. doi: 10.1016/j.rcl.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Wiering B, Ruers TJ, Oyen WJ. Role of FDG-PET in the diagnosis and treatment of colorectal liver metastases. Expert Rev Anticancer Ther. 2004;4:607–613. doi: 10.1586/14737140.4.4.607. [DOI] [PubMed] [Google Scholar]
- 17.Hustinx R. PET imaging in assessing gastrointestinal tumors. Radiol Clin North Am. 2004;42:1123–1139. doi: 10.1016/j.rcl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–540. doi: 10.1016/S1470-2045(04)01564-5. [DOI] [PubMed] [Google Scholar]
- 19.Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530–541. doi: 10.1053/j.seminoncol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 20.de Groot JW, Links TP, Jager PL, Kahraman T, Plukker JT. Impact of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in patients with biochemical evidence of recurrent or residual medullary thyroid cancer. Ann Surg Oncol. 2004;11:786–794. doi: 10.1245/ASO.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Siggelkow W, Rath W, Buell U, Zimny M. FDG PET and tumour markers in the diagnosis of recurrent and metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2004;31 Suppl 1:S118–S124. doi: 10.1007/s00259-004-1534-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Larson SM, Fazzari M, et al. Prognostic value of [18F] fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]
- 23.Andreas K, Buck MD, Sven N, Reske MD. Cellular Oigin and Molecular Mechanisms of 18F-FDG Uptake: Is There a Contribution of the Endothelium? J Nucl Med. 2004 March;45(3):461–463. [PubMed] [Google Scholar]
- 24.Daghighian F, Mazziotta JC, Hoffman EJ, Shenderov P, Eshaghian B, Siegel S, et al. Intraoperative beta probe: a device for detecting tissue labeled with positron or electron emitting isotopes during surgery. Med Phys. 1994;21(1):153–157. doi: 10.1118/1.597240. [DOI] [PubMed] [Google Scholar]