Abstract
Androgen Receptor (AR) signaling is critically important during the development and progression of prostate cancer (PCa). The AR signaling is also important in the development of castrate resistant prostate cancer (CRPC) where AR is functional even after androgen deprivation therapy (ADT); however, little is known regarding the transcriptional and functional regulation of AR in PCa. Moreover, treatment options for primary PCa for preventing the occurrence of CRPC is limited; therefore, novel strategy for direct inactivation of AR is urgently needed. In this study, we found loss of miR-34a, which targets AR, in PCa tissue specimens, especially in patients with higher Gleason grade tumors, consistent with increased expression of AR. Forced overexpression of miR-34a in PCa cell lines led to decreased expression of AR and prostate specific antigen (PSA) as well as the expression of Notch-1, another important target of miR-34a. Most importantly, BR-DIM intervention in PCa patients prior to radical prostatectomy showed re-expression of miR-34a, which was consistent with decreased expression of AR, PSA and Notch-1 in PCa tissue specimens. Moreover, BR-DIM intervention led to nuclear exclusion both in PCa cell lines and in tumor tissues. PCa cells treated with BR-DIM and 5-aza-dC resulted in the demethylation of miR-34a promoter concomitant with inhibition of AR and PSA expression in LNCaP and C4-2B cells. These results suggest, for the first time, epigenetic silencing of miR -34a in PCa, which could be reversed by BR-DIM treatment and, thus BR-DIM could be useful for the inactivation of AR in the treatment of PCa.
Keywords: BR-DIM, miR-34a, androgen receptor (AR), PSA, methylation
Introduction
Prostate cancer (PCa) is the second leading cause of cancer death in men in the United States killing over 32, 050 men in 2010 [1], which is primarily due to the emergence of Castrate Resistant Prostate Cancer (CRPC) where androgen receptor (AR) is highly functional. The mechanisms by which CRPC occurs include AR amplification and/or over-expression; gain-of-function AR mutations; intracrine androgen production; over-expression of AR co-activators; and AR activation via growth factors and cyto-kines. Together, these factors are responsible for the failure of androgen deprivation therapy (ADT) and eventual development of CRPC and bone metastasis where AR is still functional [2-4]. The mechanism(s) of treatment failure and the regulatory role of AR and its biological consequence is not fully understand although a recent study has shown that miR-34a could regulate the expression of AR in PCa cell lines [5], suggesting that the loss of miR-34a could in part be responsible for sustained expression and activity of AR in CRPC and subsequent metastasis of PCa.
MicroRNAs (miRNAs) are endogenous molecules of approximately 22-nucleotide non-coding RNAs that mediate important gene-regulatory functions by binding to the mRNAs of protein-coding genes leading to either mRNA degradation and/or translation repression. Loss or reduction of miR-34a has been found in a broad range of cancers including PCa, which in part appears to be due to aberrant CpG methylation of miR-34a promoter [6, 7]. Lodygin D et al. reported that 79.1% (19 out of 24) primary PCa exhibits CpG methylation of miR-34a promoter [7]. The miR-34a regulates many of its target genes including Notch-1 [8] and that miR-34a also inhibits PCa metastasis by targeting CD44 [9]. A recent study has shown that miR-34a could inhibit the expression of AR by binding to 3'UTR sequence of AR mRNA [5]; however, miR-34a expression and miR-34a-mediated regulation of AR in primary PCa with various Gleason grade has not been documented. Moreover, there are no reports on strategies by which re-expression of the lost miR -34a and subsequent inactivation of AR could be achieved for the inactivation of AR signaling in PCa. We have previously reported that BR-DIM could transcriptionally down-regulate the expression of AR in PCa cells and that the DHT induced nuclear translocation of AR could be inhibited by BR-DIM treatment [10].
In this study, we examined the epigenetic role of miR-34a expression in the regulation of AR in human PCa cells and in human PCa tissue specimens, and further tested the effects of BR-DIM treatment on PCa cells and PCa patients prior to radical prostatectomy. We found that the loss of miR-34a expression was partly due to methylation silencing of miR-34a promoter in human PCa cells and tumor tissue specimens resulting in the over-expression of AR, PSA and Notch-1. We also found that BR-DIM treatment of human PCa cells and patients prior to radical prostatectomy led to the re-expression of miR-34a due to demethylation of miR-34a promoter, resulting in the down-regulation of AR and nuclear exclusion, and the down-regulation of PSA and Notch-1 expression. These results are the first documenting that BR-DIM could down-regulate AR and its nuclear translocation in PCa cell lines and in human tumor tissues, which is in part due to demethylation of miR-34a resulting in its re-expression.
Materials and methods
Cell lines and culture condition
LNCaP and C4-2B cells were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 10 μmol/L Hepes, 50 units/ml Penicillin and 50 μg/ml Streptomycin. All cells were maintained in a 5% CO2-humidified atmosphere at 37°C, and genotypically characterized to support the authenticity of these cells, which was consistent with its origin.
Reagents and antibodies
Antibodies against AR, PSA and Notch-1 wee purchased from Santa Cruz (Santa Cruz, CA). Antibody to glyceraldehyde 3-phosphate dehy-drogenase (GAPDH) was purchased from Affinity BioReagents (Golden, CO). Goat anti-mouse IgG (H + L)-HRP conjugates and goat anti-rabbit IgG (H + L)-HRP conjugates were obtained from Bio-Rad (Reinach, BL). BR-DIM, a formulated DIM with higher bioavailability, was kindly provided by Dr. Michael Zeligs (abbreviated as BR-DIM or B-DIM; Bio Response, Boulder, CO) and was dissolved in DMSO to make 50 mmol/L stock solutions and stored at -20°C in multiple ali-quots for in vitro studies.
Patients and prostate tissue specimen collection
Retrospective archival pre-treatment PCa tissues and matched adjacent normal tissues were obtained from PCa patients who underwent radical prostatectomy during 2004-2010 at KCI from the Biospecimen Core of Karmanos Cancer Institute (KCI) after obtaining Wayne State University's Institutional Review Board (IRB) approval. The IRB waived the need for consent for the use of the archive samples, which was consistent with NIH guidelines, and the samples were analyzed anonymously. We also obtained PCa tissue specimens from patients who underwent clinical trial of BR-DIM (B-DIM) intervention prior to radical prostatectomy, who were newly diagnosed PCa patients from 2009-2011 at KCI and Henry Ford Health System (HFHS), Detroit, Michigan. Trial registration ID: 2007-128, which can be found in the NIH clinical trials. gov. (http://clinicaltrials.gov/show/NCT00888654). After receiving approval from Wayne State University's IRB, written informed consent was obtained from all study subjects and enrolled into the clinical trial coordinated through the Clinical Trial Office (CTO) at KCI and HFHS. Pathological features for these tissues were ascertained from microscopic evaluation of tumor slides by pathologists both at KCI and at HFHS and Gleason score (grade) was obtained in each case from the clinical database. Formalin-fixed paraffin-embedded (FFPE) tissues were used for miRNA and mRNA analysis. Patients’ clinical characteristics are shown in the Table 1.
Table 1.
Characteristics of the 127 patients underwent radical prostatectomy (RP) and 11 patients who underwent BR-DIM intervention and subsequent radical prostatectomy, and from whom prostate cancer tissue specimens were obtained
| Characteristics | Patients underwent RP | Patients underwent BR-DIM intervention and prostatectomy | |
|---|---|---|---|
| (N =127) | (N = 11) | p value | |
| Age | |||
| Year, mean(SD) | 59.5(6.5) | 58.3(4.2) | 0.4074 |
| Gleason Score | |||
| 6 | 44(34.6%) | 3(27.3%) | 0.5784 |
| 7 | 49(38.6%) | 6(55.5%) | |
| ≥8 | 34(26.8%) | 2(18.2%) | |
| PSA*(pre-intervention) | |||
| ng/ml, median(range) | 6.45(1.1-153) | 8.15(3.2-29.14) | 0.7361 |
PSA values were available from 86 patients of the 127 patients who underwent radical prostatectomy, and PSA values were available from 10 of 11 patients who underwent BR-DIM intervention and subsequent radical prostatectomy
Western blot analysis
Total cell lysates were obtained by lysing the cells in RIPA buffer. Protein concentration was determined using BCA protein assay (Pierce, Rockford, IL). Western blotting was performed as previously described [11].
miRNA and transfection
Cells were transfected with 20 and 40 nmol/L of miR-34a precursors (Ambion, Austin, TX) using DharmaFECT3 transfection reagent (DHARMACON, Lafayette, CO). After 2 days of transfection, the cells were collected and cell lysates were prepared by lysing the cells in RIPA buffer for Western blot analysis.
Real-time RT-PCR
For determining the mRNA or miRNA levels in PCa patients’ tissues, the total RNA was isolated from FFPE tissues using miRNeasy FFPE Kit (Qiagen) according to the manufacturer's instruction and the DNA was removed using an RNase-free DNAase. For the miRNA assay, 20ng of total RNA were reverse transcribed into cDNA using a Universal cDNA Synthesis Kit (Exiqon, Woburn, MA) according to the manufacturer's instruction. Real time PCR was performed using specific miR-34a primers (Exiqon) to quantify miR-34a expression by usingSYBR® Green PCR Reagents (Applied biosystems, Fostor, CA). The relative amount of miRNA was normalized to the expression of RNU1A. For testing mRNA expression, one microgram of total RNA was reverse transcribed into cDNA using a High Capacity RNA-to-cDNA Kit (Applied biosystems) according to the manufacturer's instruction. Real time PCR was performed by using SYBR® Green PCR Reagents (Applied biosystems). The relative amount of mRNA was normalized to the expression of β-actin. The sequences of primers used are shown in Table 2.
Table 2.
Primer sequences used for RT-PCR reaction
| Primer name | Sequence (5'-3') |
|---|---|
| miR-34aM-F: | GGTTTTGGGTAGGCGCGTTTC |
| m i R-34a M -R: | TCCTCATCCCCTTCACCG CCG |
| miR-34aU-F: | IIGGTTTTGGGTAGGTGTGTTTT |
| m i R-34a U -R: | AATCCTCATCCCCTTCACCACCA |
| AR-F: | TCCAGGATGCTCTACTTCGCCCC |
| AR-R: | CCGGGACTTGTGCATGCGGT |
| PSA-F: | GCAGTCTGCGGCGGTGTTCT |
| PSA-R: | TGGGCAGCTGTGAGGACCCA |
| Beta-actin-F: | CCCGCCGCCAGCTCACCATG |
| Beta-actin-R: | CGACGACGAGCGCGGCGATA |
M: methylation, U: unmethylation, F: forward, R: reverse.
Methylation-specific PCR
C4-2B and LNCaP cells were treated with 5 μM 5-Aza-2'-deoxycytidine (5-aza-dC) or 6 μM BR-DIM for 5 days. Control cells received 0.05% DMSO. Fresh 5-aza-dC, BR-DIM and DMSO were administered everyday along with a change of medium. After 5 days of treatment, genomic DNA was isolated by using the Wizard Genomic DNA Purification Kit (Promega). Then, 500 ng of genomic DNA was treated with bisulfite using the EZ DNA Methylation-Gold Kit (Zymo Research). The modified DNA was eluted with a final volume of 12 μl, and 1 μl was used for the methylation-specific real-time PCR. Methylation-specific real-time PCR reactions were carried out in a total volume of 10 μl reaction mixture including bisulfite-treated DNA, primers, SYBR Green Core Reagents (Applied Biosystems) and AmpliTaq Gold or ZymoTaq DNA Polymerase (Zymo Research) in StepOnePlus (Applied Biosystems). The sequences of primers for determining methylation of the miR-34a promoter are shown in Table 2. The PCR program was initiated by 10 min at 95°C before 40 thermal cycles, each of 15s at 95°C and 1 min at 60°C, and followed by melting curve detection to demonstrate the purity of product (only one peak). Data were analyzed according to the comparative Ct method and were normalized by GAPDH expression in input genomic DNA (unconverted genomic DNA) of each sample. The ratio of methylated and un-methylated miR-34a DNA in each sample was calculated.
Immunofluorscence and immunohistochemistry
Immunofluorescence experiments was performed as previously described [12]. Cells were viewed under confocal microscope and images were captured. For the immunohistochemical staining, formalin-fixed tumor tissue sections were used and immunohistochemical staining was performed by staining with specific primary antibody against AR, followed by 3,3'-diaminobenzidine (DAB). Sections were visualized under an Olympus microscope (Olympus, Japan) and images were captured with an attached camera linked to a computer.
Statistical methods
Experiments presented in the figures for cell line studies are representative of three or more repetitions. The data are presented as the mean and standard deviation (SD) in the bar charts. Comparisons of the continuous variables between two independent groups were made using two-tailed student's t test. For patient samples, comparisons of the continuous variables between two independent groups were made using the Wilcoxon rank sum test. For comparing the correlations between two variables, the miRNA and mRNA data from patients’ tissue specimen was log transformed before analysis. Spearman correlations were used to describe the strength of linear relationship between two variables. Contingency table was used to compare the variables such as Gleason score between patients and patients plus BR-DIM intervention by Chi-square test. All statistical tests were two sided at significance level of 0.05 unless otherwise specified.
Results
The miR-34a regulates AR expression in PCa
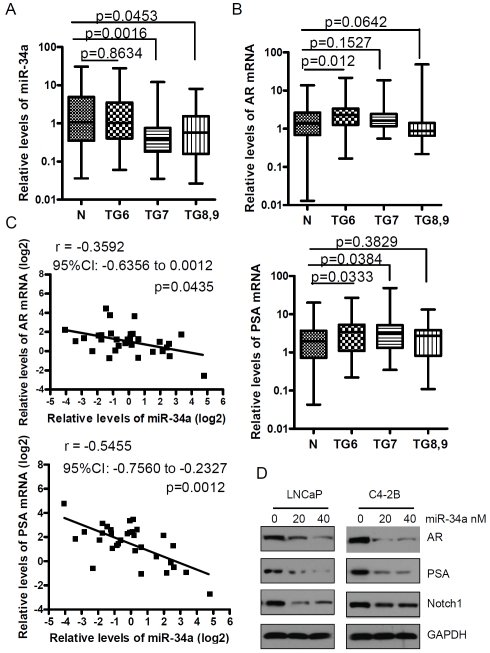
The loss of miR-34a has been shown to contribute to metastasis in PCa [9]. We sought to determine whether miR-34a was lost in the primary PCa tissue specimens. To this end, we have examined the expression status in human PCa tissues and compared with adjacent normal prostate tissues. We found that the expression of miR-34a was significantly down-regulated in PCa tissues from patients with Gleason grade 7 and greater tumors but not from patients with Gleason grade 6 tumors (Figure 1A). Since miR-34a has the binding site in the 3'UTR of AR mRNA, it suggests that miR-34a could regulate the AR expression in PCa. The results from real time RT-PCR showed that AR expression was increased in PCa tissue specimens from patients with Gleason grade 6 tumors and there was an increased trend in tumors from patients with Gleason grade 7 tumors compared with adjacent normal prostate tissues (Figure 1B, upper panel). However, a decreased trend in the expression of AR was seen in tumors from patients with Gleason grade 8 and 9 compared with adjacent normal prostate tissues (Figure 1B, upper panel, p = 0.0642). These results are consistent with data for the expression of PSA expression showing that PSA levels were up-regulated in patients’ tumors with Gleason grade 6 and 7 tumors. There was an increased trend in patients’ tumors with Gleason grade 8 and 9 tumors compared with adjacent normal prostate tissues (Figure 1B, lower panel). These results suggest that AR activity could not only depend on AR expression but could also result from cross-talk in multiple signaling pathways in higher Gleason grade tumors. Interestingly, we found that the expression of miR-34a was inversely correlated with AR and PSA expression with r = -0.3592 (95% CI: -0.6356 to 0.0012), p = 0.0435 and r = -0.5455 (95% CI: -0.7560 to -0.2327), p = 0.0012, respectively (Figure 1C). These results suggest that the loss of miR-34a could be responsible for increased expression of AR and its downstream target PSA. These results are consistent with the data from western blot analysis showing that over-expression of miR-34a by transfection of miR-34a precursors could significantly repressed AR and PSA expression in LNCaP and C4-2B cells. Moreover, we found that Notch-1 expression, which is another target of miR-34a, was also down-regulated in LNCaP and C4-2B cells transfected with miR-34a precursors (Figure 1D).
Figure 1.
The level of miR-34a expression was inversely correlated with AR and PSA expression in prostate cancer. (A) The results from real time RT-PCR showed that the levels of miR-34a were significantly down-regulated in tumors with higher Gleason grade (N: normal; TG6: tumor tissues from patients with Gleason grade 6; n = 38 for normal, n = 44 for TG6, n = 49 for TG7, n = 34 for TG8,9). (B) In the upper panel, a significant up-regulation in the expression of AR was observed in PCa tissue specimens with Gleason grade 6 (n = 34) but not in PCa specimens with Gleason grade 7 (n = 43) and 8,9 (n = 34). The lower panel showed PSA expression in the prostate cancer tissues with different Gleason grade tumors. (C) miR-34a levels were inversely correlated with AR and PSA expression in tumor specimens with Gleason grade 6. (D) Over-expression of miR-34a by transfection of cells with miR-34a precursors and incubation for 2 days showing inhibition in the expression of AR, PSA and Notch-1.
BR-DIM treatment led to re-expression of miR-34a, and down-regulated its targets, and prevented AR nuclear translocation
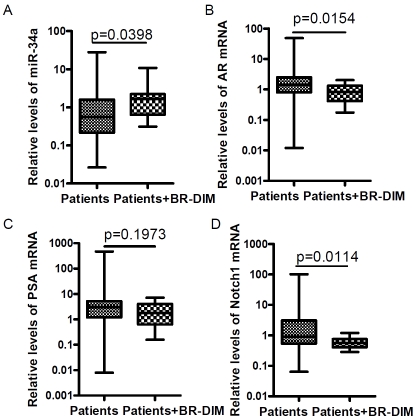
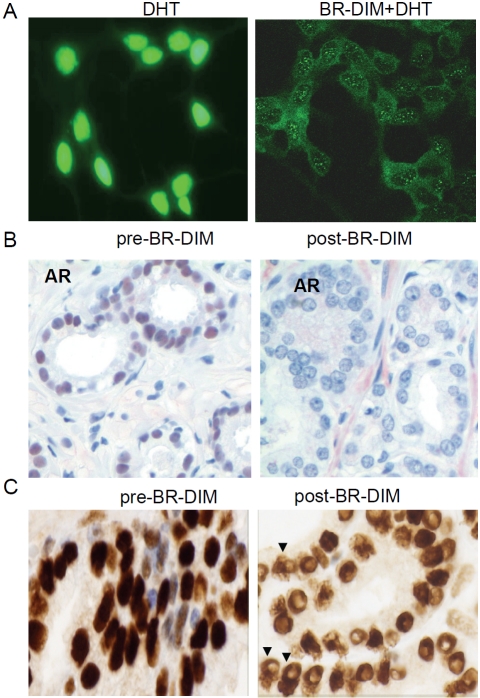
BR-DIM is the formulated DIM (BR-DIM or B-DIM) that we have used for our phase I clinical trial [13]. Our recent results showed that BR-DIM caused re-expression of miR-200b and miR -200c that are typically lost in PCa cells [14]. In the present study, the data from our on-going phase II clinical trial showed that BR-DIM treatment of PCa patients prior to radical prostatectomy led to the re-expression of miR-34a in patients’ tumor specimens after BR-DIM intervention (Figure 2A). These results are consistent with decreased expression of AR, PSA and Notch-1 (Figure 2B-D). More importantly, BR-DIM treatment down-regulated AR expression and prevented AR from nuclear translocation induced by DHT administration in LNCaP cells (Figure 3A). We also found that BR-DIM intervention in PCa patients reduced AR expression (Figure 3B) and caused nuclear exclusion of AR (Figure 3C, arrowhead) in tumor tissue specimens. Therefore, our results suggest that BR-DIM could be an important agent to re-express miR-34a and thereby inhibiting AR activity as well as inactivate Notch signaling, which would likely contribute to inhibit PCa progression.
Figure 2.
The effect of BR-DIM treatment of PCa patients prior to radical prostatectomy on miR-34a expression and its target genes in prostate tumor tissues. Eleven PCa tissue specimens were obtained from PCa patients who underwent BR-DIM intervention for 2-4 weeks prior to radical prostatectomy and used for assessing the expression of miRNA and mRNA. (A) BR-DIM intervention increased miR-34a expression, which was correlated with decreased expression of AR mRNA (B) and PSA (C) as well as Notch-1 mRNA compared with patients without BR-DIM treatment matched with age and Gleason grade.
Figure 3.
BR-DIM treatment inhibited AR expression and prevented AR from nuclear translocation. (A) Immunofluorescence staining for androgen receptor (AR) captured through confocal microscopy. LNCaP cells were cultured in RPMI1640 medium with 10% charcoal-stripped serum and kept untreated (strong nuclear staining, left panel) or treated with 25 μM BR-DIM (weaker cytoplasmic staining and nuclear exclusion, right panel) for 24 hours followed by 1 nM DHT treatment for 2 hours. (B) Immunohistochemical staining for AR using human PCa tissue specimen prior to BR-DIM intervention (diagnostic biopsy, left panel) showing strong nuclear AR staining, and after BR-DIM intervention (radical prostatectomy specimen after BR-DIM intervention, right panel) showing negative AR staining in the nuclear compartment. (C) Immunohistochemical staining for AR using human PCa tissue specimen prior to BR-DIM intervention (diagnostic biopsy, left panel) showing dark nuclear AR staining, and after BR-DIM intervention (radical prostatectomy specimen after BR-DIM intervention, right panel) showing reduced AR staining and nuclear exclusion of AR (arrowheads).
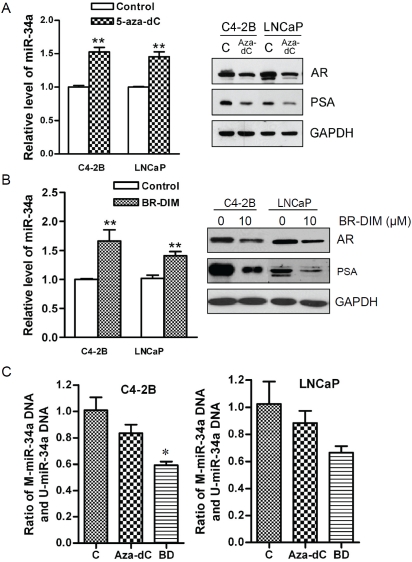
BR-DIM treatment increased the expression of miR-34a through demethylation of miR-34a promoter
Our data suggested that treatment of C4-2B and LNCaP cells with 5-aza-dC, a demethylating agent, increased miR-34a expression (Figure 4A, left panel), which is consistent with findings showing that the loss of miR-34a is indeed associated with promoter hypermethylation of miR -34a [7]. Moreover, 5-aza-dC treatment significantly inhibited the expression of AR and PSA in C4-2B and LNCaP cells (Figure 4A, right panel). These results suggest that loss of miR-34a expression is in part due to methylation of miR-34a, which could be responsible for increased expression of AR and its downstream gene PSA in human prostate cancer. Interestingly, BR-DIM treatment also increased the miR-34a expression and inhibited the expression of AR and PSA in these two cell lines similar to 5-aza-dC (Figure 4B). Therefore, our data suggest that BR-DIM could function as a demethylating agent and leads to the re-expression of miR-34a expression by de-methylating the promoter of miR-34a. As expected, treatment of cells with BR-DIM significantly reduced methylation of miR-34a promoter in C4-2B and LNCaP cells, and it was more effective than 5-aza-dC treatment (Figure 4C). These results provide mechanistic information suggesting that BR-DIM could become a novel demethylating agent for the treatment of PCa.
Figure 4.
BR-DIM regulated the expression of miR-34a, and the activity of AR pathway through demathylation. (A) The miR-34a expression was up-regulated in cells treated with 5 μM of 5-aza-dC for 5 day compared with control cells (left panel) consistent with decreased expression of AR and PSA (right panel). (B) The miR-34a expression was up-regulated in the cells treated with 6 μM of BR-DIM for 5 day compared with control cells (left panel) consistent with decreased expression of AR and PSA (right panel). (C) C4-2B and LNCaP cells were treated with 5 μM 5-aza-dC or 6 μM BR-DIM for 5 days showing decreased methylation of miR-34a promoter. (C: control, aza-dC: 5-Aza-2'-deoxycytidine, BD: BR-DIM, * p<0.05, ** p<0.01).
Discussion
Activation of androgen receptor (AR) signaling pathway is the fundamental mechanism for the development and progression of prostate cancer (PCa). Several studies have shown potential mechanism(s) by which PCa could progress to castrate resistant prostate cancer (CRPC) where AR signaling is critically important even after androgen deprivation therapy (ADT). However, very little is known regarding the transcriptional and functional regulation of AR in PCa progression. In this study, we initially examined the expression of AR in human PCa tissue specimens with variable Gleason grade tumors by quantitative real time RT-PCR. We found a significant up-regulation of AR expression in PCa tissues with Gleason grade 6 and also increased trend in Gleason grade 7 tumors, while there was some-what decrease trend in Gleason grade 8 and 9 tumors, which was associated with PSA expression. The variability of AR expression in different grade of tumors observed in this study could be due to sample size in addition to the biology of higher-grade tumors. However, our results are consistent with previous findings showing that AR expression was lower in Gleason pattern 5 (including Gleason grade 5+3, 5+4, 5+5) tumors of the primary and lymph node metastasis [15]. This could in part be due to activation of Akt and other signaling pathways, which may control the phosphorylation of AR and its stability as suggested previously [16] or it could be due to other complex mechanism of AR regulation in higher grade tumors.
A recent study has shown that miR-34a could regulate the expression of AR in PCa cell lines [5]. Hence, we examined the level of expression of miR-34a in human PCa tissue specimens and also investigated the relationship between miR-34a expression and AR expression. We found loss of miR-34a expression in Gleason grade 7, 8 and 9 tumors, and interestingly, the level of miR-34a expression was inversely correlated with AR and PSA expression in Gleason grade 6 tumors but not in Gleason grade 7, 8 and 9 tumors. These results could be due to sample size or the complexity in the biology of PCa progression. Forced over-expression of miR-34a led to the repression in the expression of AR and PSA in prostate cancer cell lines, which indeed is consistent with data obtained from human PCa tissue specimens. These results clearly suggest that miR-34a could control the expression of AR thereby affect the expression of PSA in PCa. The loss of miR-34a also found to be correlated with increased expression of Notch-1 or CD44 expressions (Notch-1 and CD44 are also the targets of miR-34a) in PCa progression where cancer stem-like cells expand to form metastasis. Therefore, re-expression of miR-34a will not only eliminate AR expression but it will also repress the expression of stem cell related markers such as Notch-1 or CD44 and thereby could inhibit PCa progression.
Interestingly, we have found that BR-DIM, an indole-3-carbinol compound, could re-express miR-34a in PCa cell lines as well as in tumor tissues after BR-DIM treatment prior to radical prostatectomy. BR-DIM is a formulated 3,3'-diindolylmetahne (DIM), which is the in vivo self-dimerized product of indole-3-carbinol (I3C) that we have used for our phase I clinical trial [13], and it is a potent agent in inhibiting the growth of PCa cells and tumors, which was found to be due to targeting multiple cellular signaling pathways [10-12, 16, 17]. Interestingly, in this study, we found that BR-DIM could demethylate the promoter of miR-34a leading to increased expression of miR-34a consistent with decreased expression of AR and PSA in PCa cells, and perhaps BR-DIM may play similar role in human PCa patients. These results are also supported by our previous findings showing that the loss of expression of miR-200 family is associated with tumor aggressiveness [18], and it is consistent with recent findings showing that the loss of miR-200 expression is mediated through methylation of miR-200 promoter [19, 20]. Therefore, BR-DIM mediated demethylation could be responsible for the re-expression of miR-34a in PCa cell lines and in tumors after BR-DIM intervention prior to radical prostatectomy resulting in the inactivation of AR signaling, which indeed could become a useful strategy for the treatment of PCa especially for the treatment of CRPC.
Acknowledgments
This work was funded by grants from the National Cancer Institute, NIH (5R01CA108535-06 and 5R01CA083695-09 to FHS, and Cancer Center Support Grant P30 CA-22453).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95:1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 4.Agoulnik IU, Weigel NL. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J Cell Biochem. 2006;99:362–372. doi: 10.1002/jcb.20811. [DOI] [PubMed] [Google Scholar]
- 5.Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71:1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 6.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 7.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 8.Li WB, Ma MW, Dong LJ, Wang F, Chen LX, Li XR. MicroRNA-34a targets notch1 and inhibits cell proliferation in glioblastoma multiforme. Cancer Biol Ther. 2011;12:477–483. doi: 10.4161/cbt.12.6.16300. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 11.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 12.Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3,3'-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3'- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 14.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 Regulates PDGF-D-Mediated Epithelial-Mesenchymal Transition, Adhesion, and Invasion of Prostate Cancer Cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischmann A, Rocha C, Schobinger S, Seiler R, Wiese B, Thalmann GN. Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases. Prostate. 2011;71:453–460. doi: 10.1002/pros.21259. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar FH, Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat Res. 2004;555:53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2011 doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, Tessema M, Leng S, Belinsky SA. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]