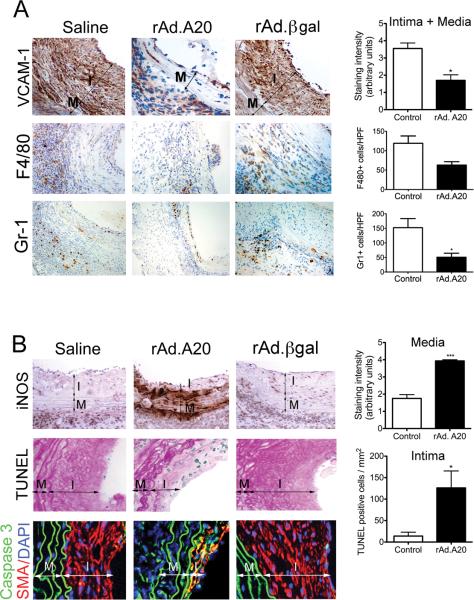
Figure. 2.
Overexpression of A20 decreases inflammation in aortic allografts while increasing apoptosis in neointimal SMC. Representative IHC photomicrographs show significantly A. Decreased VCAM-1 immunostaining in the neointimal and medial layers of rAd.A20 transduced vascular allografts at 4 weeks after transplant, as opposed to saline and rAd.βgal-treated vascular allografts; This correlated with decreased infiltration of the vascular allografts by monocytes/macrophages and neutrophils, as evaluated by immunostaining with F4/80 and Gr-1 antibodies, respectively. B. Increased iNOS immunostaining in the media of rAd.A20-treated aortic allografts correlating with C increased number of TUNEL positive (blue) neointimal SMC per mm2, as adjusted by Adobe scaling, when compared to saline and rAd.βgal treated allografts. Overlay of IF staining for smooth muscle cell α-actin SMA (red), Caspase 3 (green) and 4’,6-diamidino-2-phenylindole (DAPI, nuclear staining, blue) confirm that most TUNEL+ cells are neointimal SMC (yellow overlay). In A, B, and C, each bar represents mean±SE of immunostaining score, or number of F4/80, Gr-1, and TUNEL+ cells/HPF of 3-4 mice in rAd.A20-treated group and 4-7 mice in the control group. The control group represents combined saline and rAd.βgal treated vascular allografts (2-3 saline and 3-4 rAd.βgal-treated). I-intima, and M-media, original magnification X400 for VCAM-1, iNOS, TUNEL, and Caspase 3/SMA, and X200 for F4/80 and Gr-1. *p<0.05, ***p<0.001.