Abstract
Currently, there is only 1 published hot flash diary. This diary rates hot flash severity according to 4 categories: Mild, Moderate, Severe, and Very Severe. The descriptions of these 4 severity categories are located on a separate form from the main data form. For each 24-hour period, subjects record the number of hot flashes experienced for each of the 4 severity categories either by recollection or from a separate data source on which hot flashes have been tallied. This diary has been validated but does not conform to the FDA & EMEA Guidance for Industry. After we observed a high percentage of subjects reporting confusion when using this 4-category diary, we constructed and used a hot flash diary containing 3 severity categories that offered real-time recording of hot flashes, contained all severity definitions on the principle data form and also conformed to the FDA & EMEA Guidance for Industry. We compare these 2 diaries here and provide a sample of the 3-category diary, which has not been formally validated but is considered valid by the FDA & EMEA in support of drug approval. Either diary is acceptable for use in clinical trials.
Keywords: hot flash, hot flush, diary, clinical trial, methodology, menopause
Introduction
Hot flash clinical trials typically utilize a paper diary in order to assess subjects’ hot flash frequency and severity. The most commonly used diary in academic-initiated hot flash clinical trials assesses 4-categories of hot flash severity (Mild, Moderate, Severe, and Very Severe), the definitions of which were compiled from subjects’ perceptions.[1] For industry-initiated hot flash trials, the Food & Drug Administration (FDA) and the European Medicines Agency (EMEA) guidelines require hot flash severity to be assessed using 3-categories (Mild, Moderate, and Severe), the definitions of which were compiled from an FDA advisory committee.[2]
After we completed an initial hot flash clinical trial that used the 4-category diary[3], the Principal Investigator for this study noted that many subjects (approximately 50% from her recollection) reported confusion regarding the hot flash severity definitions included with this diary. This prompted us to develop and use a 3-category diary that conformed to FDA and EMEA guidelines. We report here on our development and use of this 3-category hot flash diary and compare its features directly with those of the 4-category diary.
Methods
In development of the 3-category diary, the definitions for Mild, Moderate, and Severe hot flashes and night sweats were taken from the FDA Guidance for Industry[2] and the NIH hot flash workshop[4], respectively, and included on each diary (Figure 1). The EMEA guidelines[5] are identical to the FDA guidelines.
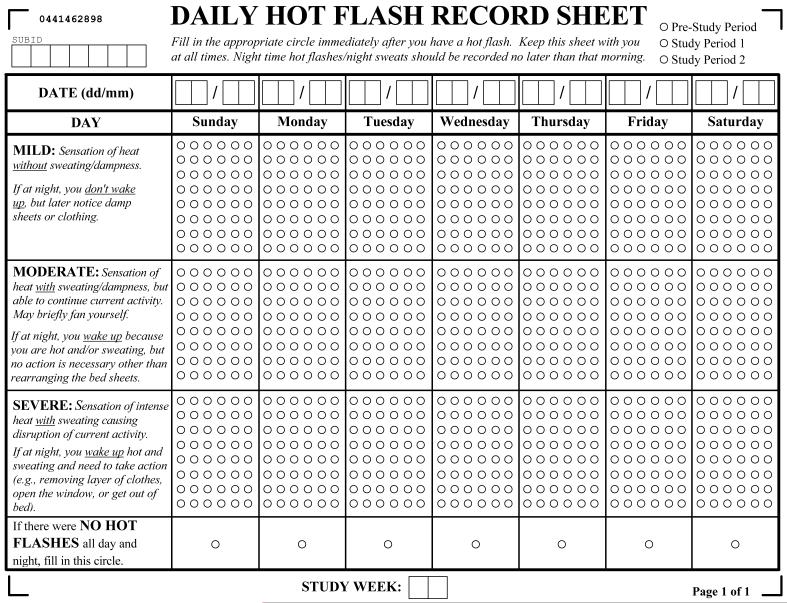
Figure 1.
3-Category Hot Flash Diary
In order to support real-time subject recording of hot flashes, 48 empty circles were placed in each hot flash severity category for each calendar day. Each circle represented the occurrence of a distinct hot flash event when filled in by the subject. Subjects were instructed to keep the diary with their person at all times possible and to record each hot flash immediately after it occurred in order to reduce errors secondary to memory recall. Night sweats were to be recorded no later than that morning upon awakening to start a new day. If no hot flashes occurred for a full 24 hours, a separate circle would need to be filled in. It was explained to subjects that this was the only way for the research team to distinguish between the absence of hot flashes and a subject’s failure to record hot flashes over a full day.
The diameter and spacing of the circles were chosen to interface with digital scanning software employing Optical Mark Recognition (OMR) to identify positively indicated hot flash episodes by the research participant.[6] In the circumstance where a subject mistakenly recorded a hot flash using an ink pen, the subject was instructed to place an X through the marked circle. During the data validation step of the scanned diaries, such events were easily detected and discounted from the record.
This 3-category diary was then used in 2 randomized controlled trials (RCTs) enrolling 151 subjects in total.[7-8] The 3-category diary (Figure 1) interfaced well with OMR scanning software. There was never an occasion when all 48 circles were filled in over a single day for any severity category among all of the diaries. Thus, there was no potential for hot flashes not being recorded due to lack of space on the diary. The Principal Investigator for these RCTs did not recall any subjects reporting confusion when using this 3-category diary.
The 4-category diary (Figure 2) and its hot flash severity definitions (Figure 3) are provided for direct comparison to the 3-category diary (Figure 1) and discussed.
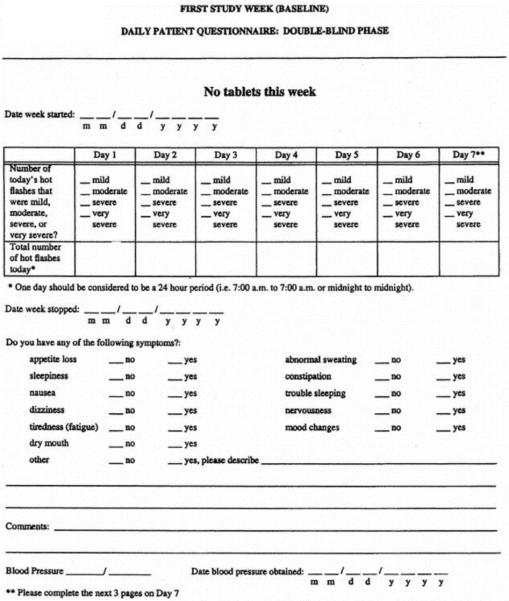
Figure 2.
4-Category Hot Flash Diary
Figure 3.
Severity Definitions for 4-Category Hot Flash Diary
Discussion
The 3-category diary provided in Figure 1 is the first published hot flash diary that conforms to the FDA & EMEA guidelines. Besides this important feature, this diary also has several other unique qualities (such as supporting the real-time recording of hot flashes, requiring subjects to confirm that a day is free of hot flashes, including the severity category definitions on the same form as where hot flashes are recorded, and being designed to interface well with OMR scanning software) that should help with the accuracy of subjects’ hot flash recording and the accuracy of the digitalized hot flash record. This diary has not been formally validated. Nevertheless, the 3-category diary is considered valid by the FDA & EMEA in support of drug approval.
In contrast, the 4-category diary does not conform to FDA & EMEA guidelines, does not support real-time hot flash recording, does not include hot flash severity definitions on the main data form, and does not interface with OMR scanning software. The 4-category diary has, however, been formally validated.[1]
Among the differences between these 2 diaries, the most significant one relates to the hot flash severity definitions. The FDA & EMEA guidelines have very simple definitions for the 3 hot flash severity categories (Figure 1). In stark contrast, the severity definitions included with the 4-category diary (Figure 3) are highly detailed, lengthy, and contain much overlap. For example: embarrassment, change in heart rate, removal of clothing, and opening of windows are all listed under both Moderate and Severe hot flash definitions for the 4-category diary.
Conclusion
The 4-category hot flash diary has the advantage of being formally validated while the 3-category diary’s main advantage is with the simplicity of the severity definitions that comply with FDA & EMEA guidelines. Either hot flash diary is acceptable for use in clinical trials.
Acknowledgments
Financial Support: RO3 HD042609 NIH/NICHD, K12 HD01332 Rochester Women’s Reproductive Health Research Career (WRHR) Center NIH/NICHD, 1K23-AT-1709-01 NIH/NCCAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Thomas Guttuso, Jr, is the inventor on US Patent 6,310,098, which is owned by the University of Rochester, covering the use of gabapentin and related compounds for treating hot flashes. The other authors have no conflicts of interest or disclaimers to report.
Contributor Information
Thomas Guttuso, Jr., University at Buffalo, 3435 Main St., Buffalo, NY USA 14214 tguttuso@buffalo.edu.
Will J. DiGrazio, University of Rochester, 601 Elmwood Ave., Rochester, NY USA 14642; Will_Digrazio@URMC.Rochester.edu.
Sireesha Y. Reddy, University of Rochester, 601 Elmwood Ave., Rochester, NY USA 14642; Sireesha_Reddy@urmc.rochester.edu.
References
- [1].Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19(23):4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- [2].U.S. Department of Health and Human Services FDA [Accessed 1 September 2010];Guidance for Industry: Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms- recommendations for clinical evaluation, 1/2003. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071643.pdf.
- [3].Reddy SY, Warner H, Guttuso T, Jr., et al. Gabapentin, Estrogen, and Placebo for Treating Hot Flushes: A Randomized Controlled Trial. Obstet Gynecol. 2006;108(1):41–8. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- [4].National Institutes of Health Assessing and Improving Measures of Hot Flashes: Summary of an NIH Workshop; 1/20/2004; [Accessed 1 September 2010]. Available at: http://nccam.nih.gov/health/hotflashes/ [Google Scholar]
- [5].European Medicines Agency EMEA [Accessed 5 October 2010];Guidline of clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. Available at: http://www.emea.europa.eu/pdfs/human/ewp/002197en.pdf.
- [6].Wahi MM, Parks DV, Skeate RC, Goldin SB. Reducing errors from the electronic transcription of data collected on paper forms: a research data case study. J Am Med Inform Assoc. 2008;15(3):386–9. doi: 10.1197/jamia.M2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guttuso T, Jr., McDermott MP, Ng P, Kieburtz K. Effect of L-methionine on hot flashes in postmenopausal women: a randomized controlled trial. Menopause. 2009;16(5):1004–8. doi: 10.1097/gme.0b013e3181a2fa76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guttuso T, McDermott MP, Su H, Kieburtz K. Effects of L-isoleucine and L-valine on hot flushes and serum homocysteine: a randomized controlled trial. Obstet Gynecol. 2008;112(1):109–15. doi: 10.1097/AOG.0b013e31817d53b6. [DOI] [PMC free article] [PubMed] [Google Scholar]