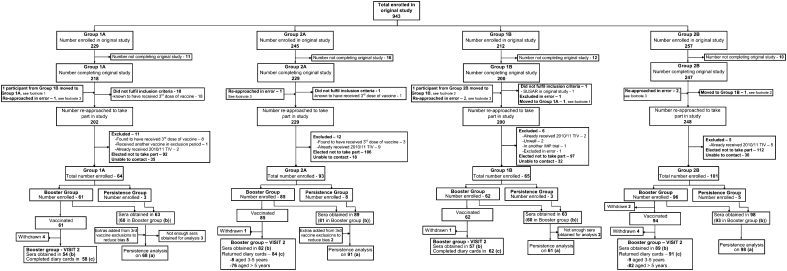
Figure 1.
Enrollment and follow-up of study participants. Groups were allocated as in the original study, first split into those receiving either the whole-virion or the AS03B-adjuvanted vaccine and then further divided into those <3 and ≥3–12 years of age at the time of the original study [3]. Annotations (a), (b), and (c) highlight the participants on whom analysis was performed: (a) indicates persistence of antibody levels; (b) and (c), immunogenicity and reactogenicity after trivalent influenza vaccine (TIV) analysis, respectively. 1In the original study, this participant accidently received the incorrect vaccine [3] (whole-virion instead of AS03B-adjuvanted vaccine, as per randomization) and was therefore moved to the whole-virion group for follow-on in this study. 2Participant was mistakenly put into the older age group in the original study but was <3 years of age by 1 day, should have been in the younger age group, and was moved there for analysis in this follow-on study [3]. 3Six participants who did not complete all of the study procedures in the original study and therefore should have been excluded from this follow-on study were approached in error [3].