Figure 1.
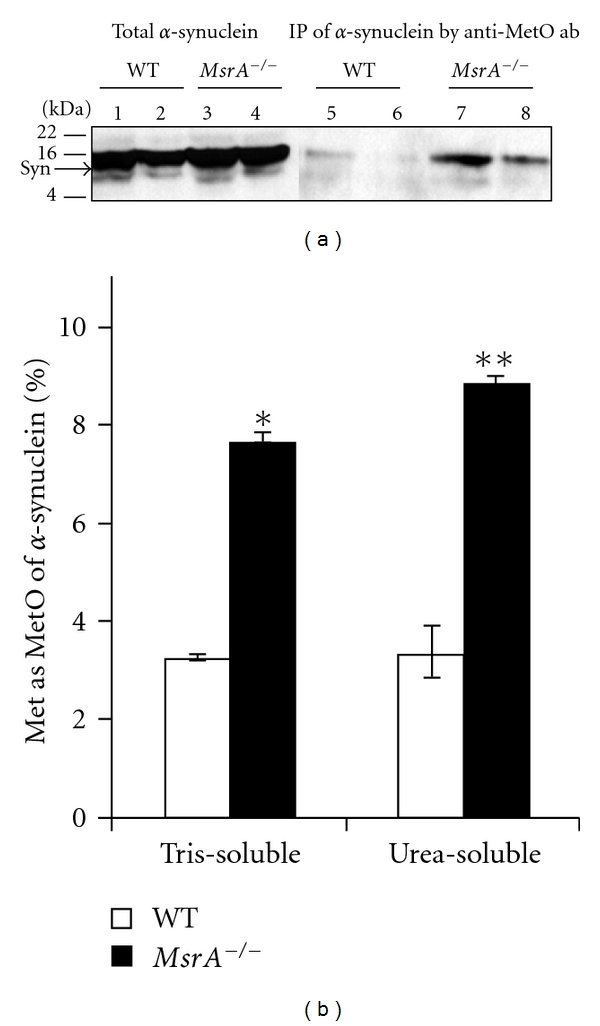
Detection of methionine oxidation of α-synuclein in MsrA −/− and wild-type control (WT) brains. (a) Brain protein extracts were prepared as Tris-soluble and urea-soluble fractions from both mouse types. Equal protein amounts of the protein extracts were either directly subjected to SDS-gel electrophoresis and Western blot analysis (Lanes 1–4) or immunoprecipitated by anti-MetO antibodies prior to the gel-loading (Lanes 5–8). Lanes 1, 3, 5, and 7 represent Tris-soluble fractions, and lanes 2, 4, 6, and 8 represent urea-soluble fractions. The Western blot analysis was probed with anti-α-synuclein antibodies as primary antibodies. The Western blot analysis is a representative blot of data generated from multiple experiments (n = 5). Syn, α-synuclein; IP, immunoprecipitation; ab, antibodies; kDA, molecular mass markers in kilo-Dalton. (b) Densitometry analysis of the α-synuclein bands detected in (a). The percent MetO values were calculated as percent ratio of the immunoprecipitant MetO-α-synuclein bands (Figure 1(a), lanes 5–8) to the corresponding bands representing total α-synuclein levels (Figure 1(a), lanes 1–4). The statistical differences between the MetO-α-synuclein levels in both mouse types were significant (n = 5; *P < .0003 and **P < .003 by t-test).
