Figure 2.
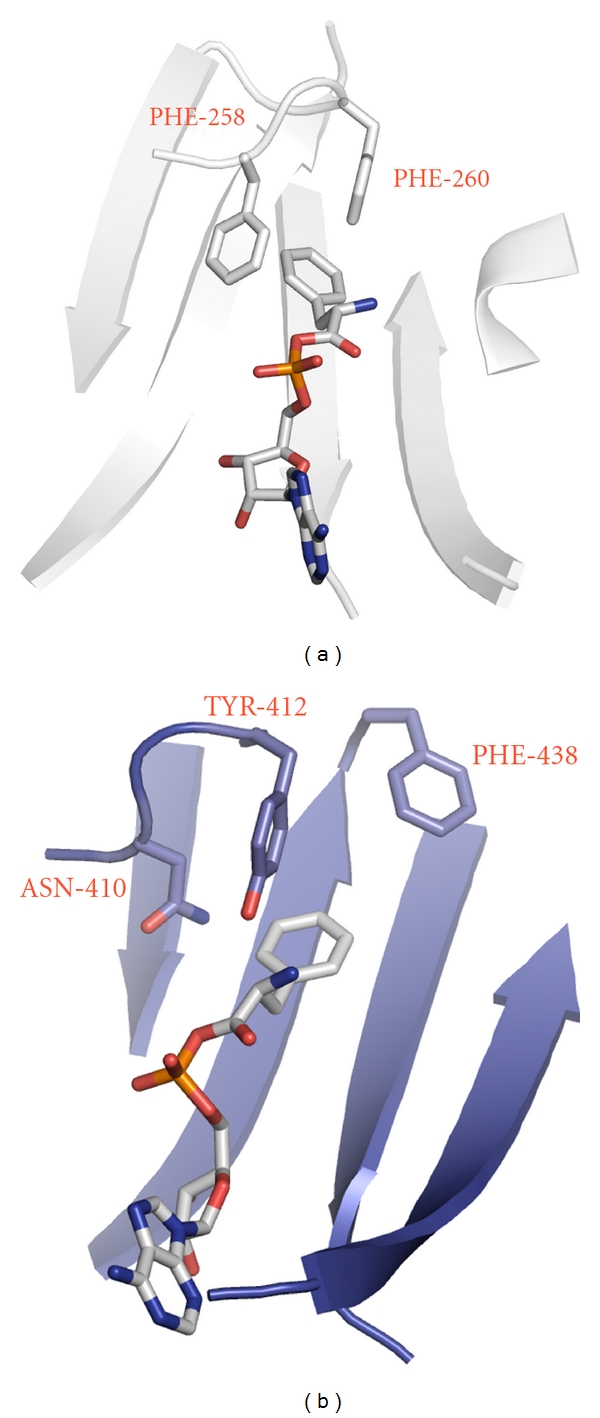
Crystal structures of ttPheRS and hcPheRS depicted in similar orientations. (a) The crystal structure of ttPheRS in the synthetic active site area complexed with bound phenylalanyl-adenylate. The principal protein residues forming “edge-to-face” interactions in aromatic triad are indicated. (b) The crystal structure of hcPheRS in the synthetic active site area. Modeling of complex with phenylalanyl-adenylate.
