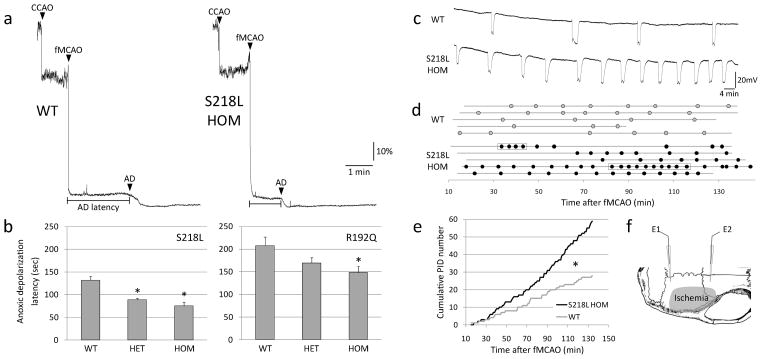
Figure 1.
Faster anoxic depolarization rates and more frequent peri-infarct depolarizations in FHM1 mutant mice. a) Representative laser Doppler tracings show CBF reduction upon common carotid artery occlusion (CCAO) followed by fMCAO. A further decline in CBF marks the onset of anoxic depolarization (AD), and is due to the vasoconstrictive effect of tissue depolarization on ischemic microvasculature18. Scale bars: vertical 10%, horizontal 1 min. b) The latency to AD was shorter in S218L and R192Q mutants (p<0.001 and p=0.035, respectively), with a trend for allele-dosage effect. *p<0.05 vs. WT. c) Representative electrophysiological tracings show more frequent PIDs in S218L HOM compared to WT. Scale bars: vertical 20 mV, horizontal 4 min. d) PIDs (round symbols shown as a function of time) occurred in S218L HOM more frequently and sometimes in clusters (rectangular boxes). Horizontal lines indicate the time of onset and end of electrophysiological recordings in each mouse (n=5 each). When calculating the average PID frequency, these minor differences in recording duration were taken into account. e) Pooled cumulative PID numbers as a function of time after fMCAO was more than doubled in S218L HOM (*p<0.001; n=5 each). f) Experimental setup showing two intracortical glass micropipettes (E1, E2) placed outside the ischemic territory to detect PIDs after fMCAO. Shaded area indicates typical distribution of CBF deficit after fMCAO.