Figure 2. Working hypothesis schematic illustrating involvement of breast-derived ASCs in the induction of fibrosis.
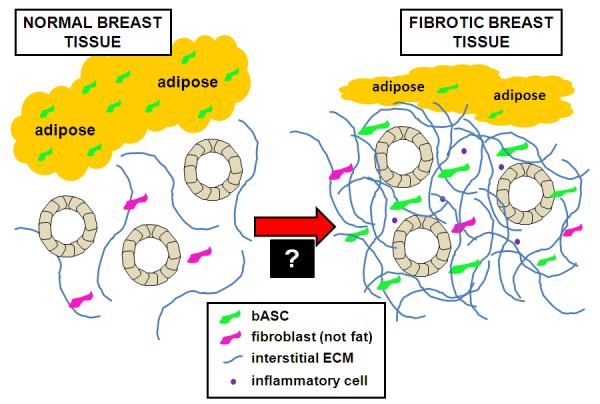
Normal breast tissue consists of glandular structures supported by a fine fibrotic stroma and an abundance of adipose tissue (on left). We propose that in response to a soluble mediator(s) derived from pre-malignant mammary epithelial cells, breast ASCs are chemoattracted from local fat stores toward the parenchyma, where they trigger a desmoplastic response characterized by their transdifferentiation into myofibroblasts, inflammation and a dense connective tissue (on right). Overall, this would result in reduced adiposity, increased connective tissue (fibrosis), and a microenvironment that could stimulate epithelial cell growth and cause genetic alterations while disrupting normal tissue homeostasis. Critical to better understanding early stage mammary carcinogenesis is validating involvement of breast ASCs in the development of fibrosis and then elucidating the mediators leading to the exodus of breast ASCs from local fat stores and altering the differentiation of these resident stem cells (as signified by the “black box” at the transition between the normal and fibrotic microenvironments).
