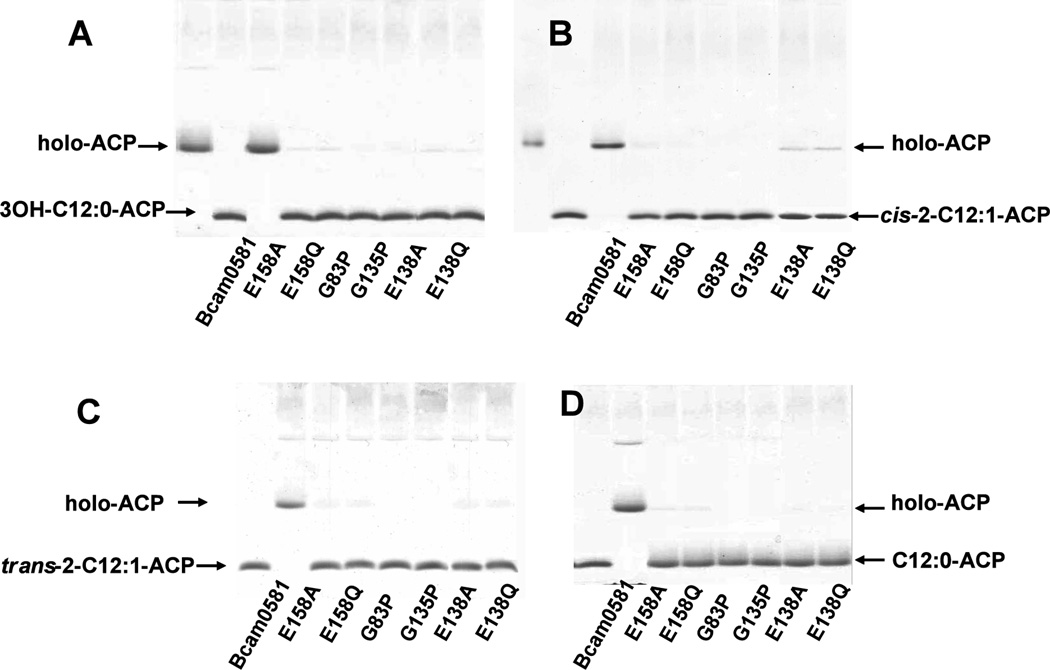
Fig. 4. Mutagenesis indicates that thioesterase activity is intrinsic to Bcam0581 and involves essential residues conserved in crotonase.
Acyl-ACPs were prepared and Bcam0581 variants were purified as described in Experimental procedures. Bcam0581 thioesterase activity assays were performed using (Panel A) 3-hydroxydodecanoyl-ACP (3-OH-C12:0-ACP), (Panel B) cis-2-dodecenoyl-ACP (cis-2-C12:1-ACP), (Panel C) trans-2-dodecenoyl-ACP (trans-2-C12:1-ACP) and (panel D) dodecanoyl-ACP (C12:0-ACP) as substrates. The reactions were initiated by the addition of 0.2 µg of wild type Bcam0581 or one of the the mutant proteins carrying amino acid substitutions E158A, E158Q, G83P, G135P, E138A or E138Q. In panels A and B the reaction mixtures were incubated at 37°C for 10 min whereas panels C and D are 30 min incubations. The reaction products were resolved by conformationally sensitive gel electrophoresis as in Fig. 3.