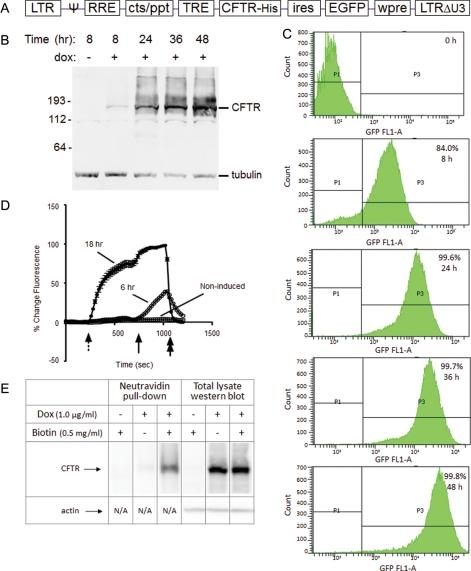
Fig. 1.
CFTR expression in GnTI− HEK293S cells. (A) The genetic structure of the P2231 lentiviral vector-based CFTR expression cassette is depicted. (B, C) Exogenous protein (CFTR and EGFP) expression in the D099 clonal cell line was analyzed by western blot (B) and flow cytometry (C) prior to and after (8, 24, 36 and 48 h) induction with dox (1 µg/ml). (D) Functionality of the recombinant CFTR protein was analyzed by the halide-sensitive fluorescent dye, SPQ. D099 cells that were either non-induced or induced with dox for 6 or 18 h, respectively, were analyzed. The dotted arrow indicates the addition of NaNO3 dequench buffer, followed by the solid arrow indicating addition of forskolin (20 µM) to maximally activate CFTR. NaI quenching buffer is resumed at the end of the experiment as a control (double arrow). (E) Surface localization of CFTR was analyzed by cell surface biotinylation. Biotinylated lysate (1500 µg) was used for the NeutrAvidin pull-down, and 30 µg lysate reserved for western blot analysis.