Abstract
Background: The size of the breast stem-cell pool could underlie the intrauterine roots of breast cancer. We studied whether breast stem cells exist in umbilical cord blood and if they correlate with hematopoietic stem-cell measurements that have been positively associated with perinatal risk factors for breast cancer.
Subjects and methods: We isolated mononuclear cells from umbilical cord blood of 170 singleton full-term pregnancies and determined, by reverse transcription polymerase chain reaction, the presence of genes of putative breast epithelial stem-cell/progenitor markers [including epithelial cell adhesion molecule (EpCAM), CD49f (α6-integrin), CD117 (c-kit receptor), CD24, and CD29 (β1-integrin)]. By immunocytochemistry, we colocalized protein expressions of EpCAM+CD49f+, CD49f+CD24+, and CD24+CD29+. We correlated concentrations of putative breast stem-cell/progenitor subpopulations, quantified by flow cytometry, with concentrations of hematopoietic stem cells.
Results: Mammary stem-cell phenotypes were identified in umbilical cord blood. The measured EpCAM+ subpopulation was positively correlated with concentrations of CD34+ and CD34+CD38− hematopoietic stem cells (both P = 0.006). Additionally, EpCAM+CD49f+ and CD49f+CD24+ subpopulations were positively correlated to the CD34+ cells (P = 0.03 and 0.008, respectively).
Conclusion: The positive association between measurable breast and hematopoietic stem cells in human umbilical cord blood suggests plausible mechanisms for a prenatal influence on breast cancer risk.
Keywords: epithelial cell adhesion molecule, flow cytometry, hematopoietic stem cell, integrins, in utero environment, prenatal origin
introduction
The hypothesis that a woman’s risk for breast cancer in the adult life is influenced already in the in utero environment [1] has implicated a role of mitogens and stem cells [2, 3]. In a large cohort of normal singleton pregnancies, we showed that the concentration of hematopoietic stem cells in umbilical cord blood is positively correlated with perinatal levels of insulin-like growth factor-1 (IGF-1) and estrogens [4, 5] and with birth weight [6], an indicator of adult life breast cancer risk [3, 7, 8]. These results are consistent with a ‘stem-cell burden and susceptibility’ hypothesis which predicts that levels of mitogens increase the number of stem cells (‘burden’), and that such stem cells are targets for genetic and/or epigenetic alterations (‘susceptibility’) that might lead to malignant transformation [3, 9, 10]. In previous studies [4–6], we used hematopoietic stem cells (defined by the CD34+, CD34+CD38−, and CD34+CD117+ cell surface markers) as a surrogate for the overall stem-cell levels. However, levels of epithelial breast stem or progenitor cells in the in utero environment would be a more biologically relevant indicator for future breast cancer risk.
Although umbilical cord blood contains hematopoietic [11] and endothelial [12, 13] stem/progenitor cells, it is challenging to assume that organ-specific breast stem cells are present in an in utero compartment far removed from the organ of interest. However, umbilical cord blood mononuclear cells (MNC) express embryonic [14] and neural [15, 16] stem-cell markers. Additionally, umbilical cord blood stem cells, possibly including mesenchymal stem cells, can be differentiated to other cell types, such as osteoblasts, chondroblasts, and adipocytes, indicating their pluripotent potential [17, 18]. Mammary phenotypes have however not been reported in human umbilical cord blood.
Here, we analyzed MNC derived from human umbilical cord blood for gene expressions of markers reported for putative breast epithelial stem cells and progenitors [19] and quantified such cell populations by flow cytometry. We report the existence of putative breast stem/progenitor cell phenotypes in umbilical cord blood and, more importantly, that the concentrations of certain breast stem/progenitor cell phenotypes found in umbilical cord blood correlate positively with those of hematopoietic stem cells.
subjects and methods
The study protocol was approved by the institutional review boards of the University of Massachusetts Medical School, Worcester, MA and Tufts Medical Center, Boston, MA.
subject recruitment and umbilical cord blood processing
Study subjects were recruited from November 2006 to November 2010 among pregnant women who delivered at the Tufts Medical Center, who were ≥18 years and human immunodeficiency virus and hepatitis B negative, with a fetus free of anomalies by ultrasound examination. For this analysis, we included only full-term (gestational age ≥37 weeks), normotensive, and singleton pregnancies. Infants were delivered according to standard obstetric practices. Umbilical cord blood was collected from the umbilical vein into a sterile bag containing 35 ml of citrate phosphate dextrose anticoagulant (Fenwal, Lake Zurich, IL). Samples were processed for MNC using a Ficoll-Paque (STEMCELL Technologies, Vancouver, Canada) density gradient within 24 h of birth as described previously [5, 6].
RT-PCR
PolyA+ messenger RNA (mRNA) was isolated from MNC using the QuickPrep Micro mRNA Purification Kit (Amersham Biosciences, Piscataway, NJ) and reversed transcribed to complementary DNA (cDNA) using the Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions. Genes of interest were detected using a set of two specific forward and reverse primers for each gene by PCR: epithelial cell adhesion molecule (EpCAM), forward 5′-TTGGTGATGAAGGCAGAAATGAATGG-3′ and reverse 5′-TGAACTAAAACACAAAGCAAGAGAAAAACCT-3′ giving a PCR product of 268 bp; CD49f (α6-integrin) [20], forward 5′-CAAGATGGCTACCCAGATAT-3′ and reverse 5′- CTGAATCTGAGAGGGAACCA-3′ giving a PCR product of 210 bp; CD117 (c-kit receptor) [21], forward 5′-AACGACACGCTGGTCCGCTG-3′ and reverse 5′-GTACACAGAACTAGACACATC-3′ giving a PCR product of 341 bp; CD24 [22], forward 5′-TGCTCCTACCCACGCAGATT-3′ and reverse 5′-GGCCAACCCAGAGTTGGAA-3′ giving a PCR product of 88 bp; CD29 (β1-integrin) [23], forward 5′-GTTACACGGCTGCTGGTCTT-3′ and reverse 5′-CTACTGCTGACTTAGGGATC-3′ giving a PCR product of 264 bp; and cyclophilin [24], forward 5′-CCACCGTGTTCTTCGACATC-3′ and reverse 5′-GGTCCAGCATTTGCCATGG-3′ giving a PCR product of 302 bp. PCR was carried out using 0.4 μM of each primer, 200 μM deoxyribonucleotide triphosphate, 1.5–3.0 mM MgCl2, 5 U AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) and ∼2 μg template cDNA in a 1X PCR buffer (50 mM KCl, 10 mM Tris-HCl pH 8.3, 0.001% gelatin). Amplifications were carried out in a thermocycler using 41 cycles of 95°C for 30 s, 55–60°C for 30 s, 72°C for 1 min, followed by a final extension at 72°C for 10 min. Amplification products were visualized under ultraviolet light after gel electrophoresis on a 2% agarose gel and staining with GelGreen Nucleic Acid Gel Stain (Botium, Hayward, CA) or ethidium bromide.
immunocytochemistry and confocal microscopy
Umbilical cord blood-derived MNC were spread and dried on to glass slides. The cells were fixed in 4% paraformaldehyde for 10 min at room temperature. After washing in 1X phosphate-buffered saline (PBS), the cells were blocked with 10% donkey serum (Millipore, Billerica, MA) in PBS for 30 min at room temperature, followed by incubation in the following primary antibodies in blocking solution overnight at 4°C: mouse anti-EpCAM (Clone E144, 1 : 100 dilution; Abcam, Cambridge, MA) and rat anti-CD49f (Clone NKI-GoH3, 1 : 200 dilution; Millipore); rat anti-CD49f and mouse anti-CD24 (Clone SN3, 1:200 dilution; Millipore); and rabbit anti-CD29 (Clone EP1041Y, 1 : 200 dilution; Millipore) and mouse anti-CD24. The cells were then washed and incubated with the appropriate secondary antibodies for 1 h at room temperature in the dark: Alexa Fluor 488 donkey antimouse, Alexa Fluor 488 donkey antirabbit, Alexa Fluor 594 donkey antirat, and/or Alexa Fluor 568 donkey antirabbit (all 1 : 200 dilution; Invitrogen/Molecular Probes, Eugene, OR). After washing, the cells were stained in DRAQ5®, 1,5-bis{[2-(di-methylamino)ethyl]amino}-4, 8-dihydroxyanthracene-9,10-dione, (Cell Signaling Technology, Danvers, MA) and coverslipped in Prolong anti fade reagent (Invitrogen/Molecular Probes). Primary antibodies were omitted for negative controls. Cellular colocalizations were examined with a True Confocal Scanning Spectrophotometer microscope (Leica Microsystems, Deerfield, IL) using excitation wavelengths 488 nm for Alexa Fluor 488, 568 nm for Alexa Fluor 568 and Alexa Fluor 594, and 633 nm for DRAQ5. Optical scanning was carried out every 0.5 μm of cell thickness by a sequential scanning method.
flow cytometric analyses
Flow cytometric analyses were carried out as described previously [5]. Briefly, 1 × 106 umbilical cord blood-derived MNC were incubated for 30 min on ice in the dark with the following fluorochrome-conjugated antibodies: anti-CD34 fluorescein isothiocyanate (FITC; Clone 581; BD BioSciences Pharmingen, San Diego, CA), anti-CD38 phycoerythrin (PE; Clone HIT2; BD Biosciences Pharmingen), anti-EpCAM FITC (Clone VU-1D9; STEMCELL Technologies, Vancouver, Canada), anti-CD49f PE (Clone GoH3; BD BioSciences Pharmingen), anti-CD117 allophycocyanin (APC; Clone YB5.B8; BD BioSciences Pharmingen), anti-CD24 FITC (Clone SN3; Antibodies-online, Aachen, Germany), anti-CD29 APC (Clone MAR4; BD BioSciences Pharmingen), or the combination of anti-CD34 FITC and anti-CD38 PE, or the combination of anti-EpCAM FITC, anti-CD49f PE, and anti-CD117 APC, or the combination of anti-CD24 FITC, anti-CD49f PE, and anti-CD29 APC. Samples treated with no antibody served as negative controls. Cells were washed, fixed with 4% paraformaldehyde, and analyzed using a FACSCalibur flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA). Hematopoietic (CD34+, CD34+CD38−) and putative breast (EpCAM+, EpCAM+CD49f+, EpCAM+CD49f+CD117+, CD49f+CD24+, CD24+CD29+, CD49f+CD24+CD29+) stem/progenitor cell subpopulations were quantified from the gated MNC population (lymphocytes and monocytes based on forward versus side light scatter) using the FlowJo software program (Tree Star, Ashland, OR). The number of cells in these populations was normalized to 103 MNC.
statistical analysis
Descriptive statistics on the characteristics of study population and laboratory data were summarized. Spearman’s rank correlation coefficients were estimated for bivariate analyses between levels of different stem-cell subpopulations. Statistical significance was set at 0.05 (two sided). STATA version 11 (StataCorp LP, College Station, TX) was used to conduct statistical analyses.
results
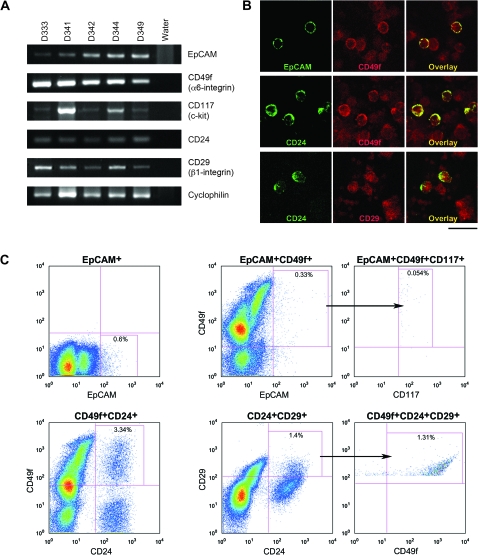
To determine whether putative breast stem/progenitor cell phenotypes were present in umbilical cord blood, we analyzed genes reported for breast stem-cell markers [19] in the MNC fraction of umbilical cord blood by RT-PCR. Because breast stem cells are considered epithelial in nature [3], we first detected the gene for EpCAM or epithelial-specific antigen (ESA), as a marker for epithelial cells [25]. Additionally, genes of putative markers for breast stem/progenitor cells, i.e. CD49f (α6-integrin), CD117 (c-kit receptor), CD24, and CD29 (β1-integrin) were detected in umbilical cord blood-derived MNC (Figure 1A).
Figure 1.
(A) Gel electrophoresis showing the detection of PCR products for epithelial cell adhesion molecule (EpCAM), CD49f (α6-integrin), CD117 (c-kit receptor), CD24, CD29 (β1-integrin), and the housekeeping gene cyclophilin from umbilical cord blood-derived mononuclear cells (MNC) from five umbilical cord blood samples (D333, D341, D342, D344, and D349). Water (last lane) was used as negative controls. (B) Double-labeled immunofluorescent confocal microscopy of umbilical cord blood-derived MNC from sample D321 showing colocalization in the overlay image of EpCAM (green) and CD49f (red) (top panel); CD24 (green) and CD49f (red) (middle panel); and CD24 (green) and CD29 (red) (bottom panel). Scale bar represents 20 μm. (C) Flow cytometric pseudocolor plots showing the detection of the EpCAM+, EpCAM+CD49f+, EpCAM+CD49f+CD117+, CD49f+CD24+, CD24+CD29+, and CD49f+CD24+CD29+ subpopulations (boxed, with percentage of cells indicated) from umbilical cord blood-derived MNC of sample N41. The arrows indicate that the triple positive population was derived from the double positive population as shown. The markers shown in the top panel have been reported in humans while the markers shown in the bottom panel have been reported in mice.
Second, we determined protein expressions by immunocytochemistry and observed colocalized staining of EpCAM+CD49f+, CD49f+CD24+, and CD24+CD29+ surface markers in umbilical cord blood-derived MNC by confocal microscopic analyses (Figure 1B). We further quantified the percentages of umbilical cord blood-derived MNC with putative markers of the different breast stem-cell subpopulations (EpCAM+CD49f+, EpCAM+CD49f+CD117+, CD49f+CD24+, CD24+CD29+, and CD49f+CD24+CD29+) in addition to EpCAM by flow cytometry (Figure 1C). Data analyses using the FlowJo software program showed that the EpCAM+ subpopulation ranged from 0.19 to 19.8 cells/1000 MNC with a mean of 3.4 ± 4.0 cells; the EpCAM+CD49f+ subpopulation ranged from 0.049 to 9.7 cells/1000 MNC with a mean of 1.7 ± 1.8 cells; the EpCAM+CD49f+CD117+ subpopulation ranged from 0.02 to 2.4 cells/1000 MNC with a mean of 0.48 ± 0.56 cells; the CD49f+CD24+ subpopulation ranged from 0 to 48.1 cells/1000 MNC with a mean of 14.7 ± 12.9 cells; the CD24+CD29+ subpopulation ranged from 0.11 to 46.2 cells/1000 MNC with a mean of 10.3 ± 9.8 cells; and the CD49f+CD24+CD29+ subpopulation ranged from 0 to 44.4 cells/1000 MNC with a mean of 8.3 ± 8.8 cells (Table 1).
Table 1.
Maternal and newborn characteristics and umbilical cord blood stem/progenitor cell counts
| Subject characteristics | N | Mean ± standard deviation or % | Range |
| Mother’s age (years) | 169 | 30.1 ± 6.2 | 19–44 |
| Parity | |||
| First | 52 | 30.8 | |
| Second | 49 | 29.0 | |
| Third | 24 | 14.2 | |
| Fourth and above | 44 | 26.0 | |
| Gestation duration (weeks) | 170 | 39.2 ± 1.2 | 37–41 |
| Newborn gender | |||
| Male | 84 | 49.4 | |
| Female | 86 | 50.6 | |
| Newborn birth weight (g) | 170 | 3,363.7 ± 510.5 | 1,973–4,917 |
| Umbilical cord blood volume (ml)a | 169 | 98.4 ± 29.4 | 45–216 |
| Umbilical cord blood stem/progenitor cell populationsb | |||
| CD34+ | 169 | 8.5 ± 7.4 | 0.0–56.7 |
| CD34+CD38− | 167 | 2.4 ± 2.5 | 0.0–23.8 |
| EpCAM+ | 112 | 3.4 ± 4.0 | 0.19–19.8 |
| EpCAM+CD49f+ | 109 | 1.7 ± 1.8 | 0.049–9.7 |
| EpCAM+CD49f+CD117+ | 32 | 0.48 ± 0.56 | 0.02–2.4 |
| CD49f+CD24+ | 56 | 14.7 ± 12.9 | 0.0–48.1 |
| CD24+CD29+ | 55 | 10.3 ± 9.8 | 0.11–46.2 |
| CD49f+CD24+CD29+ | 55 | 8.3 ± 8.8 | 0.0–44.4 |
Includes 35 ml of citrate phosphate dextrose anticoagulant.
Cell counts per 103 mononuclear cells.
EpCAM, epithelial cell adhesion molecule.
We also quantified the percentages of umbilical cord blood-derived MNC with hematopoietic stem-cell markers, i.e. CD34+ and CD34+CD38−, and carried out a rank correlation analysis between concentrations of hematopoietic and breast stem/progenitor cell subpopulations. Levels of the EpCAM+ subpopulation were positively correlated with concentrations of CD34+ and CD34+CD38− hematopoietic stem cells (both P = 0.006; Table 2). Except for the CD24+CD29+ cells, all putative breast stem/progenitor cell subpopulations were positively associated with the hematopoietic stem-cell subpopulations. Notably, the EpCAM+CD49f+ and CD49f+CD24+ subpopulations were positively and significantly correlated to the CD34+ cells (P = 0.03 and 0.008, respectively). These associations were clearer among female than among male newborns, in particular, the EpCAM+ subpopulation (Table 2).
Table 2.
Spearman’s correlation coefficients (P values in parentheses) between umbilical cord blood hematopoietic and breast stem/progenitor cell populations
| Hematopoietic stem-cell subpopulations | Breast stem/progenitor cell subpopulations |
|||||
| EpCAM+ | EpCAM+CD49f+ | EpCAM+CD49f+CD117+ | CD49f+CD24+ | CD24+CD29+ | CD49f+CD24+CD29+ | |
| All subjects (N) | 112 | 109 | 32 | 56 | 55 | 55 |
| CD34+ | 0.26 (0.006) | 0.21 (0.03) | 0.24 (0.20) | 0.35 (0.008) | 0.14 (0.32) | 0.17 (0.21) |
| CD34+CD38− | 0.26 (0.006) | 0.15 (0.12) | 0.30 (0.09) | 0.24 (0.07) | 0.01 (0.97) | 0.18 (0.19) |
| Newborn gender | ||||||
| Males (N) | 53 | 53 | 14 | 25 | 25 | 25 |
| CD34+ | 0.23 (0.37) | 0.22 (0.12) | 0.41 (0.15) | 0.33 (0.11) | 0.07 (0.76) | 0.28 (0.18) |
| CD34+CD38− | 0.02 (0.90) | 0.04 (0.77) | 0.27 (0.36) | 0.38 (0.06) | 0.09 (0.67) | 0.36 (0.08) |
| Females (N) | 59 | 56 | 18 | 31 | 30 | 30 |
| CD34+ | 0.36 (0.005) | 0.18 (0.19) | 0.03 (0.90) | 0.35 (0.05) | 0.18 (0.34) | 0.05 (0.78) |
| CD34+CD38− | 0.37 (0.004) | 0.19 (0.17) | 0.18 (0.48) | 0.19 (0.29) | −0.03 (0.88) | 0.08 (0.68) |
EpCAM, epithelial cell adhesion molecule. Statistically significant P values (P < 0.05) are shown in boldface.
discussion
To our knowledge, this is the first report of measurable breast stem cells in human umbilical cord blood. Levels of these breast stem cells correlated significantly and positively with that of hematopoietic stem cells; concentrations of hematopoietic stem cells (as surrogate measurements of overall stem-cell levels in the intrauterine environment) are correlated with umbilical cord blood plasma levels of IGF-1 and with birth weight [4–6].
Because the procurement of human cord blood samples is unpredictable, we chose to assay for putative breast stem/progenitor cells by analyzing published surface markers instead of using live cell-based methods employing dyes such as Hoechst 33342 (e.g. Invitrogen/Molecular Probes) or ALDEFLUOR® (STEMCELL Technologies) [26, 27]. Since all cells within the mammary epithelium—except for the myoepithelial cells—express EpCAM, or ESA [28, 29] and breast stem cells are considered to be epithelial in nature [3, 30], we initially examined and found the gene for EpCAM—a marker of many epithelial cells [25]—in the MNC of umbilical cord blood. In fresh, uncultured umbilical cord blood-derived MNC, we also detected other putative genes related to breast stem/progenitor cells; these include CD49f (α6-integrin), CD117 (c-kit receptor), CD24, and CD29 (β1-integrin), the latter two markers originally reported in mouse tissues [19, 31–33].
By immunostaining umbilical cord blood-derived MNC, we demonstrated the coexpression of EpCAM and CD49f (α6-integrin), a major cellular phenotype of human breast stem/progenitor cells [28, 34, 35]. The coexpressions of the proteins for CD24 and CD49f, and CD24 and CD29, indicate that mouse and human do share common markers of putative breast stem/progenitor cells.
The detection of putative breast stem/progenitor cell phenotypes suggests that there is a ‘mammary’ compartment within the umbilical cord blood. These putative breast stem/progenitor cell phenotypes in the umbilical cord blood niche could be rare but detectable by flow cytometry (Figure 1C, Table 1). Our results indicate that, at the time of birth, intrauterine conditions may sustain certain subpopulations of breast stem/progenitor cell phenotypes. It is not clear, however, how these breast phenotypes come about. Possibly, umbilical cord blood cells with breast phenotypes were derived from embryonic-like stem cells [14, 36–40] due to exposures to specific hormones or growth factors in utero, or dedifferentiated from hematopoietic stem cells as a result of epigenetic reprogramming, as had been proposed for the neural phenotypes found in umbilical cord blood [41]. These mammary phenotypes might also be differentiated from multipotent mesenchymal stem cells present in umbilical cord blood [18].
The concentration of umbilical cord blood-derived MNC carrying the epithelial EpCAM antigen was positively associated with the concentrations of hematopoietic stem-cell populations identified by the CD34 surface marker [42, 43] and the subpopulation that was positive for CD34 but negative for CD38 [44]. Notably, the EpCAM+CD49f+ and CD49f+CD24+ breast stem/progenitor cell phenotypes showed a positive correlation with the CD34+ hematopoietic stem cells. This finding supports the ‘stem-cell burden’ hypothesis [3] in predicting breast cancer risk. Nonsignificant associations might be due to the limited sample sizes for markers reported more recently and thus incorporated in later stages of our study. The inconsistent associations observed for the strictly ‘mouse’ CD24+CD29+ subpopulation suggest that such cells might not be functional mammary phenotypes in humans, although the CD49f+CD24+ subpopulation was originally reported also in mice [32]. These correlations should be reexamined in larger sample sizes, as should some of the significant associations observed in the analyses stratified by gender.
In summary, early-life exposures have been linked to risk of breast cancer in the offspring through a pathway hypothesized to involve the mammary stem-cell pool. The ‘breast stem-cell burden and susceptibility’ hypothesis is based on the assumption that breast cancer originates from mammary stem cells [3, 9, 10, 45]. The greater the number of breast stem cells, the higher the likelihood that one of these cells will undergo malignant transformation. Because mammary stem cells arise primarily during the fetal/perinatal period, the in utero environment becomes a major determinant of their number. Hence, a breast ‘stem-cell potential’—a term proposed for measurable variables that reflect the effects of intrauterine and perinatal influences on stem-cell burden and susceptibility—in umbilical cord blood might predict subsequent risk of breast cancer in the adult life. Future research will determine whether these putative populations with breast stem/progenitor phenotypes in umbilical cord blood are indeed functional mammary cells. If so, we will have a model system to understand whether genetic and/or epigenetic alterations in breast stem/progenitor cells explain fetal programming of breast cancer risk in the adult life.
funding
This work was supported by an Innovator Award from the US Department of Defense Breast Cancer Research Program, Office of the Congressionally Directed Medical Research Program (W81XWH-05-1-0314 to DT); and a grant from the US National Institute of Health (CA090902 to CCH).
disclosure
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge the contribution of the late Dr Todd Savarese during the early stages of the study. We thank Chandni Amaresan and Kimberly Cheng for their assistance during their internships at the laboratory.
References
- 1.Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet. 1990;335:939–940. doi: 10.1016/0140-6736(90)91000-z. [DOI] [PubMed] [Google Scholar]
- 2.Baik I, Becker PS, DeVito WJ, et al. Stem cells and prenatal origin of breast cancer. Cancer Causes Control. 2004;15:517–530. doi: 10.1023/B:CACO.0000036450.06092.ce. [DOI] [PubMed] [Google Scholar]
- 3.Savarese TM, Low HP, Baik I, et al. Normal breast stem cells, malignant breast stem cells and the perinatal origin of breast cancer. Stem Cell Rev. 2006;2:103–110. doi: 10.1007/s12015-006-0016-9. [DOI] [PubMed] [Google Scholar]
- 4.Baik I, DeVito WJ, Ballen K, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 2005;65:358–363. [PubMed] [Google Scholar]
- 5.Savarese TM, Strohsnitter WC, Low HP, et al. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strohsnitter WC, Savarese TM, Low HP, et al. Correlation of umbilical cord blood haematopoietic stem and progenitor cell levels with birth weight: implications for prenatal influence on cancer risk. Br J Cancer. 2008;98:660–663. doi: 10.1038/sj.bjc.6604183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:1007–1025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- 8.Park SK, Kang D, McGlynn KA, et al. Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trichopoulos D, Lagiou P, Adami HO. Towards an integrated model for breast cancer etiology: the crucial role of the number of mammary tissue-specific stem cells. Breast Cancer Res. 2005;7:13–17. doi: 10.1186/bcr966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trichopoulos D, Adami HO, Ekbom A, et al. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008;122:481–485. doi: 10.1002/ijc.23303. [DOI] [PubMed] [Google Scholar]
- 11.Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- 12.Hill JM, Zalos G, Halcox JPJ, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 13.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 14.Zuba-Surma EK, Klich I, Greco N, et al. Optimization of isolation and further characterization of umbilical cord blood-derived very small embryonic/epiblast-like stem cells (VSELs) Eur J Haematol. 2009;84:34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 15.Ha Y, Lee JE, Kim KN, et al. Intermediate filament nestin expressions in human cord blood monocytes (HCMNCs) Acta Neurochir. 2003;145:483–487. doi: 10.1007/s00701-003-0023-4. [DOI] [PubMed] [Google Scholar]
- 16.Zangiacomi V, Balon N, Maddens S, et al. Human cord blood-derived hematopoietic and neural-like stem/progenitor cells are attracted by the neurotransmitter GABA. Stem Cells Dev. 2009;18:1369–1377. doi: 10.1089/scd.2008.0367. [DOI] [PubMed] [Google Scholar]
- 17.Kögler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudreault M, Vigneault F, Gingras ME, et al. Transcriptional regulation of the human α6 integrin gene by the transcription factor NFI during corneal wound healing. Invest Ophthalmol Vis Sci. 2008;49:3758–3767. doi: 10.1167/iovs.08-1913. [DOI] [PubMed] [Google Scholar]
- 21.Rassidakis GZ, Georgakis GV, Oyarzo M, et al. Lack of c-kit (CD117) expression in CD30+ lymphomas and lymphomatoid papulosis. Mod Pathol. 2004;17:946–953. doi: 10.1038/modpathol.3800144. [DOI] [PubMed] [Google Scholar]
- 22.Kaipparettu BA, Malik S, Konduri SD, et al. Estrogen-mediated downregulation of CD24 in breast cancer cells. Int J Cancer. 2008;123:66–72. doi: 10.1002/ijc.23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchet BY, Colón M, Polotsky A, et al. Beta-1 integrin expression by human nasal chondrocytes in microcarrier spinner culture. J Biomed Mater Res. 2000;52:716–724. doi: 10.1002/1097-4636(20001215)52:4<716::aid-jbm17>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 25.Litvinov SV, Velders MP, Bakker HAM, et al. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvi AJ, Clayton H, Joshi C, et al. Functional and molecular characterization of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterizaton of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 29.Gudjonsson T, Villadsen R, Nielsen HL, et al. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GH, Chepko G. Mammary epithelial stem cells. Microsc Res Tech. 2001;52:190–203. doi: 10.1002/1097-0029(20010115)52:2<190::AID-JEMT1005>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 32.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 33.Visvader JE, Lindeman GJ. Mammary stem cells and mammopoiesis. Cancer Res. 2006;66:9798–9801. doi: 10.1158/0008-5472.CAN-06-2254. [DOI] [PubMed] [Google Scholar]
- 34.Stingl J, Raouf A, Eirew P, Eaves CJ. Deciphering the mammary epithelial cell hierarchy. Cell Cycle. 2006;5:1519–1522. doi: 10.4161/cc.5.14.2983. [DOI] [PubMed] [Google Scholar]
- 35.Villadsen R, Fridriksdottir AJ, Rønnov-Jessen L, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baal N, Reisinger K, Jahr H, et al. Expression of transcription factor Oct-4 and other embryonic genes in CD133 positive cells from human umbilical cord blood. Thromb Haemost. 2004;92:767–775. doi: 10.1160/TH04-02-0079. [DOI] [PubMed] [Google Scholar]
- 37.McGuckin CP, Forraz N, Baradez MO, et al. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005;38:245–255. doi: 10.1111/j.1365-2184.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habich A, Jurga M, Markiewicz I, et al. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp Hematol. 2006;34:914–925. doi: 10.1016/j.exphem.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp Cell Res. 2006;312:2454–2464. doi: 10.1016/j.yexcr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Halasa M, Baskiewicz-Masiuk M, Dabkowska E, Machalinski B. An efficient two-step method to purify very small embryonic-like (VSEL) stem cells from umbilical cord blood (UCB) Folia Histochem Cytobiol. 2008;46:239–243. doi: 10.2478/v10042-008-0036-1. [DOI] [PubMed] [Google Scholar]
- 41.Domanska-Janik K, Buzanska L, Lukomsja B. A novel, neural potential of non-hematopoietic human umbilical cord blood stem cells. Int J Dev Biol. 2008;52:237–248. doi: 10.1387/ijdb.072315kd. [DOI] [PubMed] [Google Scholar]
- 42.Civin CI, Strauss LC, Brovall C, et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 43.Sutherland DR, Stewart AK, Keating A. CD34 antigen: molecular features and potential clinical applications. Stem Cells. 1993;11:50–57. doi: 10.1002/stem.5530110914. [DOI] [PubMed] [Google Scholar]
- 44.Xiao M, Dooley DC. Cellular and molecular aspects of human CD34+CD38− precursors: analysis of a primitive hematopoietic population. Leuk Lymphoma. 2000;38:489–497. doi: 10.3109/10428190009059267. [DOI] [PubMed] [Google Scholar]
- 45.Ginestier C, Wicha MS. Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res. 2007;9:109–110. doi: 10.1186/bcr1741. [DOI] [PMC free article] [PubMed] [Google Scholar]