Abstract
Respiratory syncytial virus (RSV) is the most common cause of serious lower respiratory illness in infants and young children worldwide, making it a high priority for development of strategies for prevention and treatment. RSV can cause repeat infections throughout life, with serious complications in elderly and immunocompromised patients. Previous studies indicate that the RSV G protein binds through a CX3C chemokine motif to the host chemokine receptor, CX3CR1, and modulates the inflammatory immune response. In the current study, we examined the contribution of CX3CR1 to the immune response to RSV infection in mice. CX3CR1-deficient mice showed an impaired innate immune response to RSV infection, characterized by substantially decreased NK1.1+ natural killer, CD11b+, and RB6-8C5+ polymorphonuclear cell trafficking to the lung and reduced IFNγ production compared with those in wildtype control mice. Leukocytes from CX3CR1-deficient mice were poorly chemotactic toward RSV G protein and CX3CL1. These results substantiate the importance of the RSV G CX3C–CX3CR1 interaction in the innate immune response to RSV infection.
Abbreviations: BAL, bronchoalveolar lavage; RSV, respiratory syncytial virus; NK, natural killer
Respiratory syncytial virus (RSV), a member of the Paramyxoviridae family, is a ubiquitous enveloped negative-strand RNA virus that causes serious lower respiratory illness in infants and young children and complications in immunocompromised and elderly persons.3,6,7,11,12 Despite the importance of RSV as a viral respiratory pathogen, a vaccine is not currently available, and treatment options are limited. RSV encodes 11 proteins, of which the 2 major membrane glycoproteins, the RSV G (attachment) and RSV F (fusion) proteins, have important functions in the immune response to infection. Although no single cell-specific receptor has been described for the RSV G protein, a CX3C motif at amino acid positions 182 to 186 in the central conserved region has been shown to bind to the fractalkine receptor, CX3CR1; facilitate virus infection; and induce leukocyte chemotaxis.26 The CX3CR1 chemokine receptor is specific for fractalkine (CX3CL1), the only known CX3C chemokine.4,5,9,17
Fractalkine is associated with innate and inflammatory responses promoting adhesion and chemotaxis of CX3CR1-expressing cells, such as monocytes, macrophages, cytotoxic T cells, and natural killer (NK) cells.2,5,10,17,28 The importance of CX3CL1–CX3CR1 interaction in the immune response is revealed by the marked inhibition of leukocyte migration and chemotaxis associated with antiCX3CL1 or antiCX3CR1-blocking antibody treatment. RSV G protein can compete with CX3CL1 for binding to CX3CR1 and can inhibit fractalkine-mediated leukocyte chemotaxis, suggesting that the interaction of the CX3C motif of RSV G protein with CX3CR1 has immunomodulatory activities.26
The RSV G protein CX3C motif was shown to be essential in the development of enhanced pulmonary disease associated with formalin-inactivated RSV vaccination and in pulmonary expression of the proinflammatory tachykinin substance P, signifying that RSV G protein CX3C–CX3CR1 interactions may be important to the biology of RSV infection and disease pathogenesis.27 In addition, antibodies that block RSV G protein CX3C–CX3CR1 interaction protect against many of the immunomodulatory effects associated with RSV G protein, further supporting findings that the RSV G CX3C motif contributes to RSV disease.16,20,23
CX3CR1+ cells represent a predominant cytotoxic population responding to RSV infection in the lungs of BALB/c mice,14 suggesting that the CX3C–CX3CR1 interaction is vital to recruitment of cytotoxic effector cells to the lung. RSV G protein alteration of this interaction may be a mechanism to subvert antiviral immunity mediated by cytotoxic cells.14,17,28 Innate immunity provides immediate resistance to RSV infection, and the quality of the adaptive immune response relies on activation signals from the innate response. Studies have shown decreased DX5+ NK cells, RB6-8C5+ cells, and CD11b+ cell infiltration in the lungs of RSV-infected (B1 strain) mice compared with mice infected with a RSV strain that lacks the G and SH proteins, suggesting that expression of the RSV G or SH protein alters innate immune cell trafficking.27 This effect on innate immune cell trafficking may be mediated through the RSV G CX3C–CX3CR1 interaction.16,25 In the current study, we examine disease outcome after RSV infection in mice deficient for CX3CR1 and assess the contribution of CX3CR1 to innate immune-cell migration to the lungs during the disease course. We hypothesized that CX3CR1 deficiency affects the recruitment and trafficking of immune cells to the lungs of RSV-infected mice, potentially altering the antiviral response. We showed that CX3CR1 deficiency is associated with reduced innate immune cell recruitment during RSV infection but does not significantly alter the antiviral response involved in the control of viral load.
Materials and Methods
Animals.
This study was conducted in an AAALAC-accredited facility at the Centers for Disease Control and Prevention (Atlanta, GA). All procedures were reviewed, approved, and performed in accordance with the guidelines of the IACUC at the Centers for Disease Control and Prevention and the Guide for the Care and Use of Laboratory Animals.18
Female C57BL/6, BALB/c, and CX3CR1GFP (age, 6 to 8 wk) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). CX3CR1GFP mice, which lack functional CX3CR1 through replacement of the murine CX3CR1 gene with that for enhanced green fluorescent protein, were generated as previously described,19 backcrossed onto the C57BL/6 background for at least 12 generations, and maintained under SPF housing conditions. All mice were specified to be free of murine viruses (mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse rotavirus, pneumonia virus of mice, Sendai virus, lymphocytic choriomeningitis virus, murine norovirus, ectromelia virus, Hantaan virus, mouse adenovirus, mouse cytomegalovirus, respiratory enteric orphan virus 3, K virus, lactic dehydrogenase elevating virus, polyoma virus, and mouse thymic virus). In addition, the colony was specified to be free of Bordetella bronchiseptica, cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium bovis, Corynebacterium kutscheri, Helicobacter spp., Mycoplasma pulmonis, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Staphylococcus aureus, Pseudomonas spp., Streptococcus spp., and endo- and ectoparasites, according to vendor-supplied health surveillance reports. Relative humidity and temperature in the animal room were maintained between 30% to 70% and 64 to 79 °F (17.8 to 21.6 °C), respectively, under a 12:12-h light:dark cycle. Mice were housed in static polycarbonate microisolation rodent cages and fed commercial pelleted rodent chow (Lab Diet 5001, PMI, St Louis, MO) and water ad libitum. The animals were acclimated for at least 3 d before challenged with RSV infection.
Virus and infection.
The A2 strain of RSV was used in all experiments and propagated in Vero cells (ATCC, CCL 881) as previously described.27 Mice were anesthetized by intraperitoneal administration of Avertin (2% 2, 2, 2-tribromoethanol, 2% tert-amyl alcohol, 180 to 250 mg/kg) and intranasally challenged with 1.5 × 106 pfu RSV in serum-free DMEM (volume, 50 µL). At each time point, 3 to 5 mice per group were examined.
Cell collection and analysis.
At days 0 to 7 after infection, mice were anesthetized with Avertin and euthanized by exsanguination after severing of the left axillary artery. Bronchoalveolar lavage (BAL) leukocytes were harvested by lavaging the lungs 3 times by using 1 mL sterile PBS for each wash. The lungs were removed aseptically and stored at −70 °C until assay. The procedure used for extracellular staining of BAL and spleen cells was modified for microculture staining as described elsewhere.27 Briefly, BAL and splenocytes were blocked in 10% normal mouse serum in PBS with 1% bovine serum albumin for 15 min at 4 °C and then stained for 30 min at 4 °C in the dark with the appropriate combinations of fluorescein isothiocyanate-, allophycocyanin-, or phycoerythrin-labeled antiCD4, antiCD8, antiCD3, antiCD45R/B220, antiCD11b, antipolymorphonuclear cell (RB6-8C5), antiNK cell (DX5), antiNK1.1, and mouse isotype antibody controls (BD Bioscience, Mountain View, CA) diluted in staining buffer. After being washed with staining buffer, cells were acquired and the distribution of cell surface markers on 10,000 lymphocyte-gated events analyzed on a BD LSRII flow cytometer by using FACSDiva software (BD Biosciences, Mountain View, CA).
Cytokine ELISA.
The concentrations of IL4 and IFNγ in the BAL cell-free supernatant were detected by using a capture ELISA in accordance with the manufacturer's instructions (eBiosciences, San Diego, CA). Mouse CX3CL1 concentrations in the BAL cell-free supernatant were detected by using quantitative sandwich ELISA (R and D Systems, Minneapolis, MN). All samples were run in duplicate and the results expressed as pg /mL of cytokine measured.
Real-time quantitative RT-PCR.
The lungs were homogenized in PBS. Total RNA was extracted from homogenate supernatants and nasal wash by using QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instruction and stored at −80 °C. Quantitative real-time PCR was performed by using AgPath-ID OneStep RT-PCR Kit (Applied Biosystems, Foster City, CA) and an Agilent Mx3005P thermocycler (Agilent Technologies, Santa Clara, CA). The primers and probes for RSV matrix (M) gene (forward, 5′ GGC AAA TAT GGA AAC ATA GCT GAA 3′; reverse, 5′ TCT TTT TCT AGG ACA TTG TAY TGA ACA G 3′), CX3CL1 (forward, 5′ATT GGA AGA CCT TGC TTT GG 3′; reverse, 5′ GCC TCG GAA GTT GAG AGA GA 3′; probe, 5′ Fam-TCA CTG CTG CCG GTG GCT CT-BHQ-1 3′), and CX3CR1 (forward, 5′ CTG TTA TTT GGG CGA CAT TG 3′; reverse, 5′ AAC AGA TTT CCC ACC AGA CC 3′; probe, 5′ Fam-TGT CCG TCT TCT ACG CCC TCG TC-BHQ-1 3′) were obtained from the Centers for Disease Control and Prevention Biotechnology Core Facility. Consistent with previous findings,19 CX3CR1 gene expression was not detected by RT-PCR in any tissue tested (spleen, liver, brain, and peripheral mononuclear blood cells) in CX3CR1−/− mice. Threshold cycles (Ct) for each sample were calculated, and serial dilutions of known quantities (pfu) of RSV RNA were used to obtain a standard curve for quantitative real-time PCR, results are presented as pfu equivalents per milliliter (PFUe/mL). Results for CX3CR1 and CX3CL1 expression are presented as number of gene copies expressed.
Chemotaxis assay.
Spleens were harvested from wildtype C57BL/6, CX3CR1−/−, and BALB/c mice. Cell suspensions were prepared and RBC lysed by using TRIS ammonium chloride buffer (0.1 M Trizma base, 0.83% NH4Cl, pH 7.2; Sigma Aldrich, St Louis, MO). Prior to assay, cells were washed in chemotaxis buffer (RPMI1640 containing 0.1% bovine serum albumin). Cells (5× 105) in 100 μL chemotaxis buffer were loaded into the top chamber of each well of a modified Boyden chamber (filter pore size, 8 μm). The bottom of each well contained chemotaxis buffer containing either recombinant murine fractalkine (100 nM, R and D Systems), purified RSV G protein (22 μg/mL; provided by Dr Ralph A Tripp, University of Georgia, Athens, GA), murine CCL5 (100 nM, RANTES, eBiosciences), or RPMI containing 10% FBS as chemoattractants. The plates were incubated for 12 h at 37 °C, 5% CO2. For inhibition assays, antiG monoclonal antibody (225 μg/mL; clone 131-2G), antiCX3CR1 (100 nM; R and D Systems), or control antibody (100 nM; normal goat IgG) were added to the bottom chambers in the presence of either RSV G protein or CX3CL1. The optimal chemoattractant and antibody concentrations used in these assays were determined by using dose–response curves. The chemotactic index was defined as the number of viable cells migrating toward the chemoattractant divided by the number of viable cells migrating toward chemotaxis buffer, and the percentage inhibition of chemotaxis was defined as
[1 – (chemotactic index toward G protein and antibody) /
(chemotactic index toward G protein)] × 100%,
as previously described.29 All conditions were tested in triplicate. Data representative of 3 independent assays are presented.
Statistical analysis.
Statistical significance was determined by using the Student t test (Excel 2007, Microsoft Corporation, Redmond, WA), and a P value less than 0.05 was considered statistically significant. Data are shown as mean ± SEM.
Results
Expression of CX3CR1 and CX3CL1 after RSV infection.
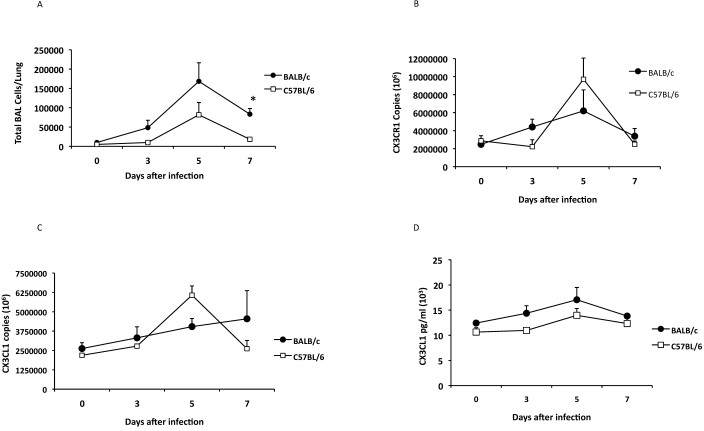
Genetic factors including genetic background may influence susceptibility to acute RSV infection in inbred mice.22,24 Although the BALB/c strain is a well-characterized model for RSV, C57BL/6 mice frequently provide background for transgenic and knockout strains of mice. To determine whether CX3CR1 and CX3CL1 expression levels after RSV infection were similar between mouse strains, BALB/c and C57BL/6 mice were intranasally infected with 1.5 × 106 pfu RSV, and pulmonary cell infiltration and expression levels of CX3CR1 and CX3CL1 were determined (Figure 1). Although the magnitude of pulmonary cell infiltration in response to RSV infection was greater in BALB/c mice as compared with C57BL/6 mice, the kinetics of BAL infiltration were similar in both strains (Figure 1 A). RSV loads, measured by real-time PCR in lung homogenates, peaked on days 3 to 5 after infection (3.3 to 4.0 log PFUe/mL) and followed similar dynamics in both C57BL/6 and BALB/c mice at all time points examined. RSV infection induced similar levels of CX3CR1 (Figure 1 B) and CX3CL1 (Figure 1 C) gene expression as well as CX3CL1 protein (Figure 1 D) in the lungs of both BALB/c and C57BL/6 mice, with peak expression levels corresponding to peak BAL cell infiltration.
Figure 1.
Expression of CX3CR1 and CX3CL1 after RSV infection. BALB/c (closed circle) and C57BL/6 mice (open square) were infected intranasally with RSV A2. (A) The time course of BAL cell recruitment after RSV infection was evaluated. The expression of (B) CX3CR1 and (C) CX3CL1 mRNA in the lungs of RSV-infected mice on days 0, 3, 5, 7 after infection is shown. (D) The concentrations of CX3CL1 in BAL-cell free supernatant were determined by ELISA. The information shown is representative data from 3 to 5 independent experiments examining 3 mice per time point (mean ± SEM; *, P < 0.05).
Impaired cell trafficking by CX3CR1-deficient leukocytes to RSV G protein and CX3CL.
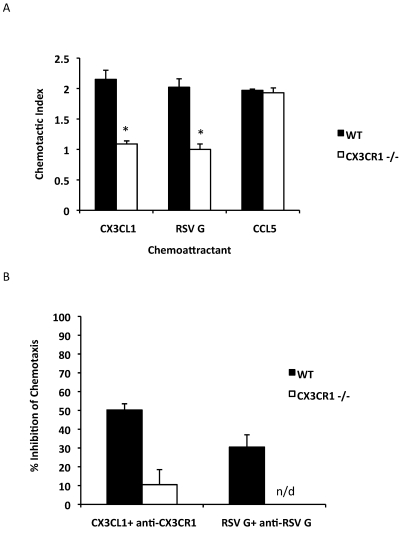
We used CX3CR1-deficient (CX3CR1−/−) mice, a well-characterized mouse model for leukocyte migration and trafficking,19 to assess the contribution of CX3CR1 to leukocyte infiltration to the lung after RSV infection. These mice lack a functional CX3CR1 and are phenotypically indistinguishable from wildtype C57BL/6 mice.19 Given that the RSV G CX3C-CX3CR1 interaction has been shown to mediate both mouse and human lymphocyte chemotaxis,15,26 we first tested whether naïve spleen leukocytes from CX3CR1−/− mice had altered RSV G-protein–mediated migration. In a modified Boyden chamber chemotaxis assay, CX3CL and RSV G protein induced similar migration in naïve leukocytes from wildtype mice (Figure 2 A). In contrast, leukocytes from CX3CR1−/− mice were not sensitive to either CX3CL1 or RSV G protein with chemotactic indices (1.1 to 1.2) comparable to those of controls that contained media alone (Figure 2 A). In fact, the ability of leukocytes from CX3CR1−/− mice to migrate toward either CX3CL1 (P = 0.02) or RSV G protein (P = 0.004) was significantly impaired compared with that of wildtype mice (Figure 2 A). However, CX3CR1−/− leukocytes migrated toward the chemokine CCL5 (RANTES) at comparable levels to those of leukocytes from wildtype mice (Figure 2 A), suggesting that the inability of CX3CR1−/− leukocytes to migrate to CX3CL1 or RSV G is specific to CX3CR1 deficiency and not a general deficiency. The ability of RSV G and CX3CL1 to induce CX3CR1+ cell migration was substantially inhibited by antiRSV G protein monoclonal antibody (clone 131-2G, 31% inhibition) and antiCX3CR1 antibody (approximately 50% inhibition, P = 0.009; Figure 2 B), consistent with previous findings.26 No appreciable inhibitory effects were detected with either antiRSV G monoclonal antibody or antiCX3CR1 antibody on CX3CR1−/− leukocytes (Figure 2 B).
Figure 2.
Spleen leukocytes from CX3CR1−/− mice fail to migrate to RSV G protein or CX3CL1 but not to CCL5. (A) Chemotaxis of leukocytes from CX3CR1−/− (white bars) and wildtype (WT, black bars) C57BL/6 mice to recombinant CX3CL1, purified RSV G protein, and CCL5 (RANTES) was assessed. (B) To inhibit CX3CL1- and RSV G–protein-mediated cell chemotaxis, antiCX3CR1 antibody and antiRSV G monoclonal antibody (131-2G) were added to chambers containing either CX3CL1 or RSV G protein, and the percentage inhibition of chemotaxis was determined. *, P < 0.05; n/d, not detected.
Pulmonary inflammatory response to RSV infection in the lungs of CX3CR1-deficient mice.
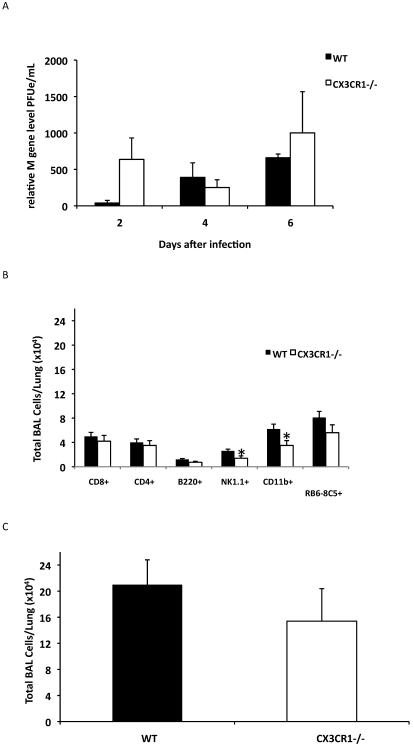
We next addressed the association between RSV G CX3C–CX3CR1 interaction and pulmonary cell trafficking in CX3CR1−/− and wildtype mice infected with RSV. Viral loads in the lungs of CX3CR1−/− and wildtype mice, as measured by real-time PCR at days 2 to 6 after infection, revealed no significant difference between strains (Figure 3 A), suggesting that loss of CX3CR1 alone did not significantly alter the ability of the host to control RSV infection. The deficiency in CX3CR1 did not significantly alter CD4+, CD8+, or B220+ cells trafficking on day 4 or day 6 (Figure 3 B; P = 0.082, P = 0.078, and P = 0.151, respectively) after infection, suggesting that this deficiency had minimal effect on adaptive immune T and B cell recruitment. On day 4 after infection, CX3CR1−/− mice showed a trend (P = 0.10) for decreased total pulmonary cell infiltration compared wildtype mice (Figure 3 C). However, RSV-infected CX3CR1−/− mice exhibited a 43% to 48% reduction in NK1.1+ NK cells (P = 0.032) and CD11b+ cells (P = 0.04) and a modest decrease (30.2% reduction) in RB6-8C5+ polymorphonuclear cells compared with those in wildtype mice (Figure 3 B), suggesting that CX3C–CX3CR1 interaction is important in innate immune cell recruitment to the lungs after RSV infection. These findings are consistent with earlier observations that numbers of DX5+ NK and RB6-8C5+ cells were decreased in RSV-infected mice treated with antiCX3CR1 antibody.16 Of note, the percentages of NK1.1+ (approximately 2%), CD11b+ (approximately 14%), and RB6-8C5+ (less than 1%) cell infiltration in uninfected CX3CR1−/− mice were comparable to those of wildtype C57BL/6 controls (approximately 2%, approximately 17%, and less than 1%, respectively), indicating that altered pulmonary cell infiltration was associated with RSV infection.
Figure 3.
CX3CR1−/− mice have impaired innate cell recruitment after RSV infection. CX3CR1−/− (white bars) and wildtype (black bars) mice were infected with RSV A2. (A) The viral load in the lungs of RSV-infected mice was determined by quantitative RT-PCR. (B) BAL cell types and (C) total BAL cell numbers on day 4 after infection. The information shown is representative data from 3 to 5 independent experiments examining 3 mice per time point (mean ± SEM; *, P < 0.05).
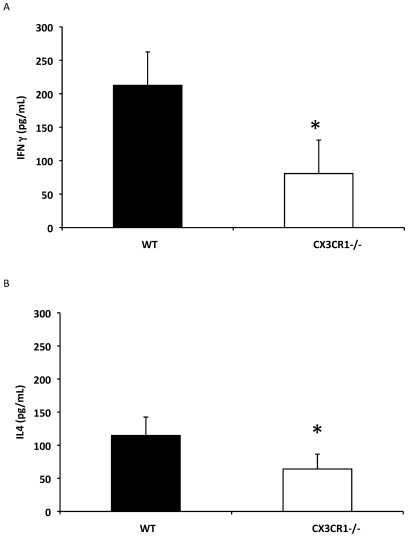
To evaluate the effect of CX3CR1 deficiency on aspects of the inflammatory response associated with RSV infection, cell-free BAL concentrations of IFNγ and IL4 in cell-free BAL supernatants were determined. Proinflammatory IFNγ production in BAL fluids on day 6 after infection was dramatically and significantly (P = 0.017) decreased (Figure 4 A), and similarly reduced IL4 levels (P = 0.03; Figure 4B) were detected in RSV-infected CX3CR1−/− mice as compared with wildtype mice.
Figure 4.
CX3CR1−/− mice have impaired cytokine production after RSV infection. CX3CR1−/− (white bars) and wildtype (black bars) mice were infected with RSV A2. (A) IFNγ and (B) IL4 levels in cell-free BAL supernatant were measured by ELISA on day 6 after infection. The information shown is representative data from 3 to 5 independent experiments examining 3 mice per time point (mean ± SEM; *, P < 0.05).
Discussion
Fractalkine (CX3CL1), the natural ligand for CX3CR1, is important in many aspects of innate immunity functioning as both a chemoattractant (soluble form) and an adhesion molecule (membrane-bound form) mediating the capture and recruitment of CX3CR1+ cells which include monocytes, macrophages, NK cells, and cytotoxic T lymphocytes.2,9 In the current study, we show that RSV infection was associated with increased mRNA expression of CX3CL1 and CX3CR1 and that peak expression coincided with peak pulmonary leukocyte infiltration, which was similar in both BALB/c (H-2Dd) and C57BL/6 (H-2Db) mice. These results suggest that levels of CX3CR1 and CX3CL1 mRNA expression are not significantly influenced by host genetic background. During RSV infection, the RSV G CX3C–CX3CR1 interaction has at least 2 important roles during the course of RSV infection. First, the interaction of the RSV G protein CX3C motif with the host's chemokine receptor, CX3CR1, may modify the host's immune response by disrupting CX3CL1-mediated responses, altering cytokine expression and the activation and recruitment of antiviral CX3CR1+ Th1-type cells.15,25,27 Second, the RSV G CX3C–CX3CR1 interaction has been shown to contribute to enhanced disease associated with FI-RSV vaccination.16 Our current findings, together with studies showing that a polymorphism in the CX3CR1 gene is associated with occurrence of RSV bronchiolitis in humans, led us to hypothesize that CX3CR1 deficiency would alter RSV G CX3C–CX3CR1 interaction and the subsequent RSV-associated immune response.
CX3CR1−/− C57BL/6 mice challenged with RSV exhibited impaired pulmonary NK1.1+ NK, CD11b+ cell, and, to a lesser extent, RB6-8C5+ cell infiltration. Although CX3CR1 deficiency only mildly decreased overall pulmonary leukocyte infiltration, our finding that CX3CR1 deficiency decreased certain cell subsets is similar to previous findings obtained by using antiCX3CR1 antibody treatment in FI-RSV–enhanced disease.16 In that study, antiCX3CR1 antibody treatment was associated with decreased trafficking of DX5+, RB6-8C5+, and CD3+ cells after RSV challenge in FI-RSV–vaccinated mice, suggesting that CX3C–CX3CR1 interaction is important in pulmonary recruitment of these cell types.16 Because it is a ligand for CX3CR1, RSV G protein can mimic CX3CL activities and induce leukocyte chemotaxis of naïve mouse leukocytes in vitro.26 As expected, naïve leukocytes from our CX3CR1−/− mice did not migrate to either CX3CL or purified RSV G protein, whereas both chemoattractants induced similar chemotactic indices in wildtype mice. Further, antiRSV G monoclonal antibody (131-2G), which reacts at amino acids 1 to 173 proximal to the central conserved region and blocks CX3C-CX3CR1 interaction,1 inhibited RSV G-protein–induced migration of leukocytes from wildtype mice but not of leukocytes from CX3CR1−/− mice. These data confirm that CX3CR1−/− mice do not have a functional CX3CR1 and support the hypothesis that RSV G protein and CX3CL1 induce cell migration through similar mechanisms that both involve CX3CR1.
In addition to altered leukocyte trafficking, CX3CR1 deficiency in our mice was linked to significantly decreased IFNγ expression in the lungs of RSV-infected mice compared with wildtype control mice, suggesting that the majority of IFNγ response after RSV infection is produced by the CX3CR1+ cell population. This conclusion is consistent with previous studies showing that the majority of IFNγ response at days 6 and 12 after RSV infection is produced by class-I-restricted, RSV-specific CX3CR1+ cells.15 Taken together, these results further support the importance of RSV G CX3C–CX3CR1 interaction in mediating leukocyte migration and modulating the inflammatory response after RSV infection.
The RSV G CX3C–CX3CR1 interaction facilitates virus infection. Both the CX3CR1 and glycosaminoglycan are considered receptors for RSV infection,26 and the majority of RSV G protein binding to the host cells occurs through interactions between the heparin binding domains and glycosaminoglycans.8 In CX3CR1−/− mice, RSV likely used interaction between the RSV G heparin binding domains and glycosaminoglycans on cells or alternatives to facilitate infection. Therefore, our data do not rule out CX3CR1 as a receptor for RSV. Moreover, we detected no significant differences in virus load between CX3CR1−/− mice and wildtype C57BL/6 mice. Further, the ability to clear virus did not appear to differ markedly between mouse strains, suggesting that loss of CX3CR1 alone did not alter the ability of the host to control RSV infection.
Soluble and membrane-bound forms of RSV G protein are both expressed during RSV infection. Soluble RSV G protein and CX3CL1 compete for CX3CR1 binding, resulting in disruption of CX3CL1-mediated responses and altered activation or recruitment of antiviral CX3CR1+ Th1-type cells.25 CX3CR1+ cells represent a key cytotoxic population responding to RSV infection in the lungs of BALB/c mice; however, RSV-specific cytotoxic responses have been demonstrated in RSV-specific CX3CR1– splenocytes.14 In mice and humans, NK cells with high CX3CR1 expression are more cytotoxic than are NK cells with negative or low receptor expression13,21 and preferentially migrate toward fractalkine.21 In the current study, the numbers of CD8+ and CD4+ cells from CX3CR1−/− and wildtype mice showed no significant differences at the time points examined. However, in the absence of CX3CR1+ cytotoxic cells, CX3CR1−/− mice may generate a less efficient antiviral response that can still control infection. Further phenotypic and functional characterization of NK and T cells in RSV-infected CX3CR1−/− deficient mice are needed.
In conclusion, we demonstrated that in the absence of CX3CR1, there is altered pulmonary trafficking of innate immune cells (NK1.1+ cells, CD11b+ cells, and RB6-8C5+ polymorphonuclear cells) and Th1-type cytokine expression by BAL cells after RSV infection. These results provide additional evidence to support the importance of RSV G CX3C–CX3CR1 interaction in the immune response to RSV infection and highlight the importance of intervention strategies designed to disrupt this interaction.
Acknowledgments
We thank Dr Ralph A Tripp (University of Georgia) for his comments and critique and Dr Tamas Nagy (University of Georgia) for his technical assistance with histopathologic evaluation. We also thank Dr Nathaniel Powell (Centers for Disease Control and Prevention) and Yvonne Reed (Centers for Disease Control and Prevention) for their assistance in this protocol. This research was supported by program funds provided by the Centers for Disease Control and Prevention and the Centers for Disease Control and Prevention Laboratory Animal Medicine Residency Program. Additional funds were made available to EGB through an appointment to the Research Participation Program at Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education (ORISE), through an interagency agreement between the US Department of Energy and Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Anderson LJ, Bingham P, Hierholzer C. 1988. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol 62:4232–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640–644 [DOI] [PubMed] [Google Scholar]
- 3.Campbell AP, Chien JW, Kuypers J, Englund JA, Wald A, Guthrie KA, Corey L, Boeckh M. 2010. Respiratory virus pneumonia after hematopietic cell transplantation (HCT): assocations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clincial outcomes of HCT. J Infect Dis 201:1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combadiere C, Gao J, Tiffany HL, Murphy PM. 1998. Gene cloning, RNA distribution, and functional expression of mCX3CR1, a mouse chemotactic receptor for the CX3C chemokine fractalkine. Biochem Biophys Res Commun 253:728–732 [DOI] [PubMed] [Google Scholar]
- 5.Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM. 1998. Identification of CX3CR1 A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV1. J Biol Chem 273:23799–23804 [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 7.Falsey AR, Walsh EE. 2005. Respiratory syncytial virus infection in elderly adults. Drugs Aging 22:577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman SA, Hendry RM, Beeler JA. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virology 73:6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. 1998. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 188:1413–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foussat A, Coulomb-L'Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. 2000. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol 30:87–97 [DOI] [PubMed] [Google Scholar]
- 11.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283:499–505 [DOI] [PubMed] [Google Scholar]
- 12.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann I, Unterwalder N, Cardona AE, Meisel C, Zipp F, Ransohoff RM, Infante-Duarte C. 2011. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology 133:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. 2006. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 176:1600–1608 [DOI] [PubMed] [Google Scholar]
- 15.Harcourt JL, Karron RA, Tripp RA. 2004. Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C–CX3CR1 binding and leukocyte chemotaxis. J Infect Dis 190:1936–1940 [DOI] [PubMed] [Google Scholar]
- 16.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. 2003. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C–CX3CR1 interaction and expression of substance P. J Virol 77:9831–9844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. 1997. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91:521–530 [DOI] [PubMed] [Google Scholar]
- 18. Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20:4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. 2009. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol 90:1119–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. 2002. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol 168:6173–6180 [DOI] [PubMed] [Google Scholar]
- 22.Prince GA, Horswood RL, Berndt J, Suffin SC, Chanock RM. 1979. Respiratory syncytial virus infectionin inbred mice. Infect Immun 26:764–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. 2010. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in RSV challenged naïve and formalin-inactivated RSV immunized BALB/c mice. J Virol 84:9632–9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark JM, McDowell SA, Koenigsknecht V, Prows DR, Leikauf JE, LeVine AM, Leikauf GD. 2002. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol 67:92–100 [DOI] [PubMed] [Google Scholar]
- 25.Tripp RA. 2004. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 17:165–181 [DOI] [PubMed] [Google Scholar]
- 26.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738 [DOI] [PubMed] [Google Scholar]
- 27.Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol 73:7099–7107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. 2000. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol 164:4055–4062 [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA. 2010. Vaccination to induce antibodies blocking the CX3C–CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]