Abstract
Obesity causes innate immune dysfunction, contributing to increased disease risk. Weight loss from a combination of caloric restriction and exercise is the most effective treatment of obesity. We compared forced and voluntary exercise as weight-loss treatments in diet-induced obese (DIO) mice and assessed the effects of weight loss on monocyte concentration and cell-surface expression of Toll-like receptor (TLR) 2, TLR4, CD80, and CD86. DIO CD1 male mice were allocated randomly to 1 of 3 groups (n = 6 per group): voluntary wheel running (VEX); forced treadmill running (FEX); and sedentary (S). A fourth (control) group (CN, n = 6) of nonDIO mice was included also. During the 8-wk weight-loss treatment, all 4 groups consumed a low-fat (10% fat) diet. Nonlethal saphenous vein blood samples collected at baseline, week 4, and week 8 were analyzed by flow cytometry to assess monocyte concentration and functional receptor expression. The VEX and FEX groups lost significantly more body weight (36% and 27%, respectively) over the 8 wk of treatment than did other groups. VEX mice ran 4.4 times more than did FEX animals. VEX mice had higher monocyte concentrations (48% and 58%, respectively) than did the CN and FEX groups. Compared with baseline, week 8 cell-surface expression of TLR2 (22%), TLR4 (33%), and CD86 (18%) was increased in VEX mice. At week 4, CD80 expression was 42% greater for VEX than S mice. The present study confirms that short-term exercise and low-fat diet consumption cause significant weight loss and altered immune profiles.
Abbreviations: CN, control diet and normal activity; DIO, diet-induced obesity; FEX, forced wheel running; S, sedentary; TLR, Toll-like receptor; VEX, voluntary wheel running
Diet-induced obesity (DIO) is a leading health concern for the world's population, in light of effects on healthcare costs, morbidity, and mortality.1,4,28 Although a regimen that combines caloric restriction with exercise appears to be the most effective treatment for DIO,16-18 research has not fully defined what aspects of exercise are most important. Mouse models for treating DIO represent an excellent opportunity to test various types of exercise that could be used for treating humans.
In addition to weight loss, exercise has been shown to elicit antiinflammatory properties that can be measured as changes in blood monocyte concentration and functional receptor expression.8,15,17 We previously found that forced treadmill running during DIO did not prevent weight gain or alterations in blood monocytes.8 Others have speculated that forced exercise in mice is an effective treatment modality due to unnecessary psychologic stress, thereby preventing the antiinflammatory effects that typically are associated with exercise training.2
Although many ways to assess innate immune status are available, our laboratory and others have focused on monocyte concentration and functional receptor expression.8,15,17 Our laboratory has monitored the Toll-like receptor (TLR) pathway as a means to track changes in systemic inflammatory capacity.17-19 The TLR pathway is considered to be a key mediator of the innate immune response14 and systemic inflammation.10,17 Subjects eating high-fat diets show increased gene expression of TLR2 and TLR4 on blood monocytes with increases in blood fatty acids and hyperglycemia.22,26 Cell-surface expression of TLR4 and TLR2 in vitro on blood monocytes increases in response to increased blood glucose concentration.5 Elevated fasting glucose and high cholesterol levels, which often occur during weight gain, are known risk factors for several obesity-associated diseases; therefore increases in TLR4 and TLR2 are believed to be part of the inflammatory process that leads to disease.
In addition, TLR4 has been shown to influence body composition in mice. TLR4-knockout mice fed a high-fat diet gained less body fat than did wildtype mice.12 In another study,26 TLR4 knockout mice weighed 15% less on average than did wildtype controls after 8-wk of high-fat feeding. In addition, adipocytes were 30% smaller in the TLR4-knockout mice, indicating that lack of TLR4 affected fat accumulation.26 Furthermore, TLR4-knockout mice had decreased expression of the proinflammatory cytokines IL6 and TNFα,26 consistent with other studies that reported a reduction in the inflammatory status of mice that lacked TLR4.14,24 Considering these results together led us to hypothesize that TLR may play a role in weight gain and inflammatory status that leads to disease progression.
The costimulatory molecules CD80 and CD86 also are known to be part of the inflammatory process. Both CD80 and CD86 are present on antigen presenting cells and interact with T cells to prime them against antigenic attack.21 These molecules have been shown to increase with inflammatory states such as cardiovascular disease and obesity7 and may decrease after an antiinflammatory process such as exercise.15
Here we evaluated weight loss in DIO mice treated for 8 wk with voluntary compared with forced exercise. We also evaluated the effects of these regimens on blood monocyte concentration and the expression of TLR2, TLR4, CD80, and CD86. We hypothesized that, in mice that became obese after consuming high-fat diet, voluntary exercise combined with a low-fat diet would cause greater alterations in inflammation than would low-fat diet either alone or combined with forced exercise.
Materials and Methods
Animals.
All procedures described in this report were reviewed and approved by the University of Houston IACUC (Houston, TX). CD1 male mice were purchased from Charles River Laboratories (Wilmington, MA), tattooed (AIMS Tattoo System, Binghampton, NY) on the tail for identification purposes, and kept on a reverse 12:12-h light:dark cycle (lights off, 1000). Mice were 12 to 14 wk of age at the start of the weight-gain phase and 64 to 66 wk of age at the start of the active intervention stage. Mice were housed individually in standard microisolation caging (Tecniplast, Exton, PA) for the duration of the study. Body weight and food consumption were measured weekly by using a digital scale (MyWeigh, Phoenix, AZ). Food was presented to mice on wire cage lids. Twice each week, food was changed and residual (food left in cage lid) and wasted (food in bottom of cage) were combined and weighed. Food consumed was determined by subtracting residual or wasted food from the total amount of food given.
Determination of sample size.
The sample size of 24 for this current study was selected based on an a priori sample size calculation that used the outcome variable with the smallest effect size (in this case, monocyte TLR4 expression).8 This calculation indicated that, if using a repeated-measures model, we needed a minimum of 5 mice per treatment group to detect statistical significant differences between the 3 DIO treatment groups.
DIO in mice.
DIO was established in CD1 mice by using a weight-gain protocol developed in our laboratory and documented elsewhere.3 Briefly, to elicit DIO, mice were provided ad libitum access to a high-fat, high-calorie diet (5.24 kcal/g: 60% from fat, 20% from protein, and 20% from carbohydrates) for 12 mo; CN mice were provided ad libitum access to a low-fat, reduced-calorie diet (3.85 kcal/g; 10% from fat, 20% from protein, and 70% from carbohydrates) for 12 mo. Both diets were purchased from Research Diets (New Brunswick, NJ). The fat source in both diets was soybean oil and lard (cholesterol content, 0.95 mg/g). All groups had ad libitum access to water.
Group assignment.
After the 12-mo DIO phase, each mouse was assigned randomly to 1 of 3 treatment groups (n = 6 per group) according to diet composition and exercise treatment: voluntary exercise (VEX), forced treadmill exercise (FEX), and sedentary (S). After assignment, mice in the 3 treatment groups began consuming the low-fat diet, on which CN mice remained. S and CN mice were restricted to normal daily activity within their cages; these mice were sedentary in the sense that they did not undergo any structured physical activity. Body weight and food intake were measured weekly by using a digital scale. All groups were provided ad libitum access to food and water for the duration of the weight-loss phase.
Forced exercise treatment.
In the FEX group, mice ran on a motorized treadmill (Columbus Instruments, Columbus, OH) 5 d each week (Monday through Friday). Each exercise session consisted of 60 min of treadmill running (22 m/min) during the first 2 h of the dark cycle (1000 to 1200). Treadmills have been successful as a means of aerobic intervention in murine research, because mice will run with little to no motivation.8,20 If mice did become unwilling to run, they were pushed gently by hand to encourage them.
Voluntary exercise treatment.
The mice in the VEX group were housed individually in commercially available wheel-running cages (Tecniplast) with 24 h access to an exercise wheel 5 d each week (Monday through Friday), to match the days on which the FEX group ran. The exercise wheel (circumference, 73 cm) was connected to a computer, which recorded the time of day and number of wheel rotations completed by individual mice. From these data, we calculated the distance run each day.
Nonlethal blood collection.
Blood collection was completed at baseline, week 4, and week 8 of the study by using a nonlethal technique.8 Briefly, during the last 2 h of the light cycle (0700 to 0900) and after overnight (at least 8 h) fasting and abstention (more than 48 h) from exercise, mice were placed in a modified 50-mL centrifuge tube with their hindlimbs accessible. A hindlimb was shaved with electric clippers, a thin layer of petroleum jelly was applied to the skin, and the saphenous vein was punctured by using a 5-mm sterile lancet (Medipoint, Mineola, NY). Approximately 60 µL of blood was collected into an appropriately labeled lithium-heparin–treated microvette capillary tube (Sarstedt, Nümbrecht, Germany).
Flow cytometry staining and acquisition.
Two-color flow cytometry was used to analyze monocyte concentration and cell-surface expression of TLR2, TLR4, CD80, and CD86 as described previously.3 All antibodies and solutions were purchased from the same vendor (eBioscience, San Diego, CA) unless otherwise noted. Briefly, an aliquot of whole blood (5 µL) was combined with an FC receptor blocking cocktail (1:50 dilution of unlabeled antiCD16/32) to minimize nonspecific binding. A 2-color antibody cocktail (PECy5-labeled CD14 and either PE-labeled TLR2, TLR4, CD80, or CD86) was added to each blood sample and incubated for 30 min in the dark at room temperature. An isotype-control tube was used to identify a negative population. PMT voltages were set by using the isotype controls to ensure that negative populations were within the first logarithmic decade for all colors. RBC were lysed with a commercial lysing buffer and washed prior to a final suspension in staining buffer (100 μL) and 1% paraformaldehyde solution (100 μL; Electron Microscopy Sciences, Hatfield, PA). Signals were acquired within 48 h of fixation on a flow cytometer (Guava EasyCyte Mini, Millipore, Billerica, MA) equipped with a 20-mW, 488-nm solid-state laser. Uncompensated FCS 2.0 data files were exported for further analysis.
Flow cytometry analysis.
Acquired FCS data files were electronically compensated for spectral overlap and analyzed offline by using FCS Express (version 3, De Novo Software, Los Angeles, CA). Monocytes were identified and quantified by using a side scatter (SSC) compared with CD14 dot plot. Cell-surface expression of TLR2, TLR4, CD80, or CD86 as geometric mean fluorescence intensity was quantified by using individual histograms gated on CD14+ events. In the Results section, percentages were used to illustrate the changes, but data were not analyzed as percentage change.
Statistical analysis.
All statistical analyses were completed by using SPSS version 17.0 (SPSS, Chicago, IL). Data was examined before formal analysis to assess their normal distribution. Nonnormal data were log-transformed prior to analysis. Body weight, food intake, and distance run were analyzed by using 4 (group) × 8 (time) ANOVA with repeated measures on the second factor. Monocyte measurements were analyzed by using 4 (group) × 3 (time) ANOVA with repeated measures on the second factor. Statistical significance was set at a P value of less than 0.05. Location of significant differences was completed by using a Tukey post hoc test. Data were presented as either group mean ± 1 SD or percentage change from baseline, depending on the nature of the outcome measurement.
Results
Body weight.
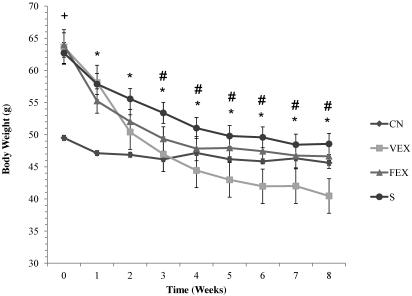
DIO mice gained an average of 53% of initial body weight, compared with an average of 35% for CN, thus making body weight at baseline 28% lower in CN than in FEX, VEX, and S mice (F24,144 = 16.024, P < 0.0001; Figure 1). This effect was due to the 12-mo pretreatment phase, when CN mice consumed a low-fat diet but the FEX, VEX, and S groups consumed a high-fat diet. Body weight decreased each week during the weight-loss phase, leading to a significant time effect (F8,144 = 190.414, P < 0.0001). Significant group × time interactions (F24,144 = 16.024, P < 0.0001) emerged for VEX and FEX mice between baseline and weeks 3 through 8. By week 8, VEX and FEX had lost 36% and 27% of baseline body weight, respectively.
Figure 1.
Body weight (g). *, Significant main effect for time; #, significant group × time interaction between VEX and FEX from baseline; +, significant group effects from CN. Significance defined as a P value of 0.05 or less.
Caloric intake.
Caloric intake did not differ statistically. During the 8-wk weight-loss period, CN mice consumed 798 ± 5 kcal each, VEX mice consumed 812 ± 29 kcal each, FEX mice consumed 753 ± 20 kcal each, and S mice consumed 694 ± 8 kcal each.
Distance run.
During the 8-wk treatment, VEX mice ran significantly (F1,9 = 29.521, P < 0.0001) farther than did FEX mice. Whereas VEX mice ran 25,405 ± 8179 m each week per mouse, FEX mice ran 5728 ± 1796 m each week per mouse.
Monocyte concentration.
Significant (F3,18 = 6.244, P = 0.004) group effects were found between VEX (0.06 ± 0.05 cells/mL) and both CN (0.03 ± 0.01 cells/mL) and FEX (0.03 ± 0.01 cells/mL) groups but not S (0.09 ± 0.08 cells/mL).
TLR2 and TLR4 expression.
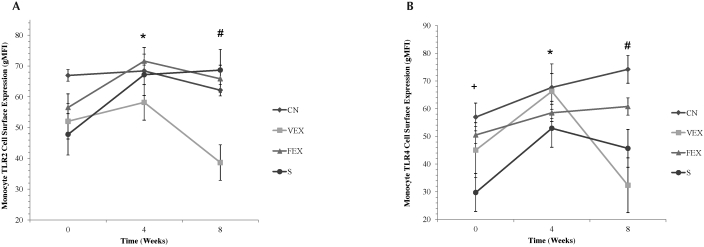
A significant group × time interaction (F6,36 = 4.206, P = 0.003) was found for cell-surface TLR2 expression (Figure 2). At week 8, VEX had 60% to 78% less TLR2 expression than did the other 3 groups. A similar significant group × time interaction (F6,36 = 3.394, P = 0.009) was found for cell-surface TLR4 expression. At week 8, TLR4 expression in VEX was 130% lower than in CN and 88% lower than in FEX. In addition, significant (F3,18 = 8.189, P = 0.001) group interaction was present for TLR4 expression at baseline. TLR4 expression in CN was 20% higher than in VEX and 47% higher than in S at baseline.
Figure 2.
Cell-surface expression (geometric mean fluorescence intensity, gMFI) of (A) TLR2 and (B) TLR4 in monocytes. *, Significant main effect for time; #, significant group × time interaction; +, significant group effects from CN. Significance defined as a P value of 0.05 or less.
CD80 and CD86 expression.
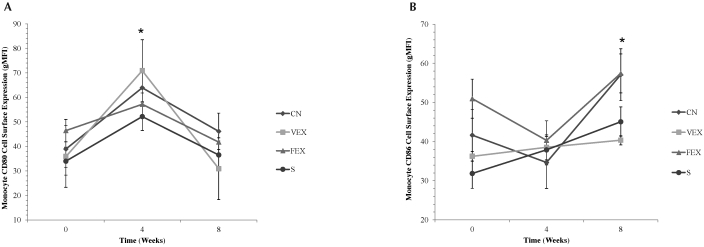
A significant (F2,34 = 30.832, P < 0.001) time effect emerged for monocyte cell-surface expression of CD80 (Figure 3); that is, expression at baseline was significantly different than that at week 4, and expression at week 4 was significantly different than that at week 8. In addition, significant (F2,34 = 11.599, P < 0.001) time effect was present in cell-surface expression of CD86. No significant group differences or interactions were found for either CD80 or CD86.
Figure 3.
Cell-surface expression (geometric mean fluorescence intensity, gMFI) of (A) CD80 and (B) CD86 in monocytes. *, Significant main effect for time. Significance defined as a P value of 0.05 or less.
Discussion
Compared with forced exercise in mice, voluntary exercise caused greater reductions in body weight and monocyte TLR2 and TLR4 cell-surface expression. In fact, the forced exercise treatment actually increased monocyte TLR2 and TLR4 expression, a counterproductive effect in terms of the intended goal of reversing DIO.5,22 Similar increases in TLR2 and TLR4 expression occurred in mice that lost weight but did not exercise, suggesting that forced treadmill running is not an effective weight loss treatment in mice. We did not observe any treatment-specific improvements in monocyte CD80 or CD86 expression, suggesting that regardless of the mode, exercise does not necessarily improve these outcome measures. More research is needed to determine whether the observed changes in monocytes are strictly a function of differential weight loss that occurred. Controlling for weight loss differences statistically does not fully account for their biologic and immunologic implications.
All mice in the intervention group lost weight, but those treated with voluntary exercise lost significantly more body weight than did mice that underwent either forced exercise or sedentary treatments. Differential weight loss is likely due to the differences in exercise volume and resultant caloric expenditure between groups. In the present study, mice that voluntarily exercised ran almost 5 times farther, thus resulting in greater caloric expenditure and weight loss, than did those forced to exercise. How to administer exercise-training programs in mice is a topic of considerable debate in the scientific literature. Some researchers believe forced exercise may cause unnecessary physical stress to an animal, resulting in subsequent increases in inflammatory status in and of itself.20 Other investigators have argued that voluntary exercise provides a stress-free stimulus that forced exercise does not. For example, when allowed free access to a running wheel, C57BL/6 mice exercise an average of 10,000 m daily.6 This volume of exercise would be considered extreme in humans, but the activity patterns of humans and rodents differ significantly. In terms of inflammatory status, 16 wk of free wheel running reduced plasma TNFα and increased antioxidant capacity compared with mice that completed treadmill running. Free-wheel mice ran an average of 22 km/wk, which is similar to the 25 km/wk average for VEX mice in the current study.11 Clearly, mice will exercise significantly more when they are provided free access to a running wheel than when they are forced to run.
In addition to treatment-associated differences in weight loss, we noted different responses in monocytes. The VEX treatment resulted in a greater decrease in monocyte TLR2 and TLR4 expression than did either the FEX or S treatments. In fact, forced exercise actually induced a slight increase in cell surface expression of these markers, although the increase was less pronounced than that in the S group. In addition, these changes may be attributed to the magnitude of the energy expenditure, which is greatest during voluntary exercise, followed by forced exercise, and least during sedentary activity. In light of previous studies, the differential ability of exercise training to attenuate increased expression of TLR2 and TLR4 is likely due to the volume of training (that is, distance per week). In another study,8 forced exercise similar in volume to that of the FEX treatment in the current study during a period of diet-induced weight gain blunted increases in monocyte TLR4 expression, suggesting that forced exercise was sufficient to oppose changes associated with diet-induced weight gain. In the present study, however, increased exercise volume (as seen with voluntary exercise) was needed to counter the short-term disturbances of weight loss.
Previous literature has shown that increases in TLR2 accompany increases in triglycerides and blood glucose, which are common in sedentary subjects eating a high-fat diet.5 Even though weight loss occurred in S group mice, perhaps the extensive period of high-fat feeding was too long for them to achieve the attributes of the low-fat controls. The changes in weight loss and inflammatory status we noted in mice are consistent with findings from humans after exercise training.10 Because neither the present study nor other published studies have matched forced and voluntary exercise groups on training volume, we are unsure whether the volume or the mode of exercise actually caused the differences we found. Future research should compare forced and voluntary exercise at equal exercise volumes to determine the underlying mechanism.
The results of the current study add another factor supporting the idea that exercise, voluntary exercise in particular, can attenuate inflammatory markers in mice. Although a previous study2 measured inflammatory markers in the adipose tissue (TNFα, MCP1, and so forth), we noted a decrease in the expression of monocyte receptors that may be indicative of inflammatory potential; these cells migrate to the peripheral tissues and mature into resident macrophages. In a previous study,11 16 wk of voluntary exercise in a range similar to that in the present study was associated with a decrease in plasma TNFα, an outcome that is consistent with the antiinflammatory action of exercise. An increase of proinflammatory markers in the blood likely translates to a proinflammatory state in the tissues as well.27 Decreased TNFα mRNA expression with chronic (12 to 20 m/min for 60 min/d on 5 d/wk for 16 wk) exercise training in mice.13 In humans, as little as 9 wk of exercise training was associated with a decrease in systemic inflammation as well as decreased monocyte expression of TLR2 and TLR4.9,23,25 Therefore, the antiinflammatory effect in the present study is consistent with previous reports involving mouse and human subjects.
Whereas the changes in monocyte TLR2 and TLR4 expression in response to weight loss and exercise training were clear, those in CD80 and CD86 expression was less obvious. TLR2 and TLR4 play roles in inflammation, whereas CD80 and CD86 affect T-cell priming. These different physiologic roles in the immune system may be related to the varied responses we noted. In the current study, monocyte expression of CD80 and CD86 did not change significantly. To our knowledge, the current study is the first to examine monocyte expression of CD80 and CD86 after a period of weight loss with exercise training. More research is needed to fully understand how body weight and exercise training influence the expression of these markers in mice. Upregulation of costimulatory molecules for the subsequent production of cytokines is a key function of TLR.15 In humans, stimulation with ligands for TLR2 and TLR4 led to increases in CD86 expression and decreases in CD80 expression after strenuous exercise in humans.15 These previous results15 contrast with those of the present study, a difference that may reflect the subject population, that is, humans compared with mice. Further investigation into changes in monocyte expression in mice is warranted. To our knowledge, this current study is one of the first to examine changes in CD80 and CD86 expression by using a longitudinal mouse model.
In conclusion, the differences between voluntary and forced exercise treatments in mice most likely can be attributed to differences in the volume of training, given that VEX mice exercised more than did FEX mice. From an applied standpoint, the current study demonstrates the importance of exercise at mitigating transient disruptions in monocytes that may occur during the treatment of DIO. More research is needed to understand the subtle differences between forced and voluntary exercise modes, but the present study provides critical data demonstrating that exercise during weight loss is beneficial, decreasing inflammatory biomarkers, regardless of mode. Our current findings as well as future studies likely will provide additional insight regarding improved treatment for obesity.
Acknowledgments
This study was funded in part by a Student Development Research Award from the Texas Chapter of the American College of Sports Medicine, a Graduate Student Research Award from the University of Houston, and a Summer Fellowship Award from the Texas Obesity Research Center to KCC. The authors declare no conflict of interest.
References
- 1.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. 2006. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12 [PubMed] [Google Scholar]
- 2.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. 2008. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295:E586–E594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breslin WL, Strohacker K, Carpenter KC, Esposito L, McFarlin BK. 2010. Weight gain in response to high-fat feeding in CD1 male mice. Lab Anim 44:231–237 [DOI] [PubMed] [Google Scholar]
- 4.Bulcao C, Ferreira SR, Giuffrida FM, Ribeiro-Filho FF. 2006. The new adipose tissue and adipocytokines. Curr Diabetes Rev 2:19–28 [DOI] [PubMed] [Google Scholar]
- 5.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. 2008. High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bono JP, Adlam D, Paterson DJ, Channon KM. 2006. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol 290:R926–R934 [DOI] [PubMed] [Google Scholar]
- 7.Dopheide JF, Sester U, Schlitt A, Horstick G, Rupprecht HJ, Munzel T, Blankenberg S. 2007. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80, and CD86 in vitro. Coron Artery Dis 18:523–531 [DOI] [PubMed] [Google Scholar]
- 8.Esposito LM, Simpson RJ, Strohacker K, Carpenter KC, McFarlin BK. 2010. Defining a longitudinal survival model to examine forced treadmill running as a countermeasure for diet-induced weight gain. Lab Anim 44:305–311 [DOI] [PubMed] [Google Scholar]
- 9.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. 2003. Antiinflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42:861–868 [DOI] [PubMed] [Google Scholar]
- 10.Gleeson M, McFarlin B, Flynn M. 2006. Exercise and Toll-like receptors. Exerc Immunol Rev 12:34–53 [PubMed] [Google Scholar]
- 11.Hoffman-Goetz L, Pervaiz N, Guan J. 2009. Voluntary exercise training in mice increases the expression of antioxidant enzymes and decreases the expression of TNFα in intestinal lymphocytes. Brain Behav Immun 23:498–506 [DOI] [PubMed] [Google Scholar]
- 12.Johnson GB, Riggs BL, Platt JL. 2004. A genetic basis for the ‘Adonis’ phenotype of low adiposity and strong bones. FASEB J 18:1282–1284 [DOI] [PubMed] [Google Scholar]
- 13.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. 2010. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat–diet-induced obese mice. Exerc Immunol Rev 16:105–118 [PubMed] [Google Scholar]
- 14.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. 2007. Toll-like receptor 4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100:1589–1596 [DOI] [PubMed] [Google Scholar]
- 15.Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. 2005. The physiological regulation of Toll-like receptor expression and function in humans. J Physiol 563:945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur N, Pedersen BK. 2008. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm 2008:109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. 2006. Physical activity status, but not age, influences inflammatory biomarkers and Toll-like receptor 4. J Gerontol A Biol Sci Med Sci 61:388–393 [DOI] [PubMed] [Google Scholar]
- 18.McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. 2004. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc 36:1876–1883 [DOI] [PubMed] [Google Scholar]
- 19.McFarlin BK, Johnston CA, Tyler C, Hutchison AT, Kueht ML, Reeves R, Foreyt JP. 2007. Inflammatory markers are elevated in overweight Mexican–American children. Int J Pediatr Obes 2:235–241 [DOI] [PubMed] [Google Scholar]
- 20.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. 2000. Treadmill running produces both positive and negative physiological adaptations in Sprague–Dawley rats. Am J Physiol Regul Integr Comp Physiol 279:R1321–R1329 [DOI] [PubMed] [Google Scholar]
- 21.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. 2009. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS ONE 4:e6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. 2006. TLR4 links innate immunity and fatty-acid–induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. 2005. Influence of exercise training and age on CD14+ cell-surface expression of Toll-like receptor 2 and 4. Brain Behav Immun 19:389–397 [DOI] [PubMed] [Google Scholar]
- 24.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. 2007. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun 354:45–49 [DOI] [PubMed] [Google Scholar]
- 25.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. 2008. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the antiinflammatory influence of exercise? J Leukoc Biol 84:1271–1278 [DOI] [PubMed] [Google Scholar]
- 26.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. 2007. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 27.Woods J, Lu Q, Ceddia MA, Lowder T. 2000. Special feature for the Olympics. Effects of exercise on the immune system: exercise-induced modulation of macrophage function. Immunol Cell Biol 78:545–553 [DOI] [PubMed] [Google Scholar]
- 28.You T, Nicklas BJ. 2006. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr Diabetes Rev 2:29–37 [DOI] [PubMed] [Google Scholar]