Abstract
During early development in vertebrates, pluripotent cells are generated from the neural crest and migrate according to their presumptive fate. In birds and mammals, one of the progeny cells, melanoblasts, generally migrate through a dorsolateral route of the trunk region and differentiate to melanocytes. However, Silky is an exceptional chicken in which numerous melanoblasts travel via a ventral pathway and disperse into internal organs. Finally, these ectopic melanocytes induce heavy dermal and visceral melanization known as Fibromelanosis (Fm). To identify the genetic basis of this phenotype, we confirmed the mode of inheritance of Fm as autosomal dominant and then performed linkage analysis with microsatellite markers and sequence-tagged site markers. Using 85 backcross progeny from crossing Black Minorca chickens (BM-C) with F1 individuals between White Silky (WS) and BM-C Fm was located on 10.2–11.7 Mb of chicken chromosome 20. In addition, we noticed a DNA marker that all Silky chickens and the F1 individuals showed heterozygous genotyping patterns, suggesting gene duplication in the Fm region. By quantitative real-time PCR assay, Silky line-specific gene duplication was detected as an ∼130-kb interval. It contained five genes including endothelin 3 (EDN3), which encoded a potent mitogen for melanoblasts/melanocytes. EDN3 with another three of these duplicated genes in Silky chickens expressed almost twofold of those in BM-C. Present results strongly suggest that the increase of the expression levels resulting from the gene duplication in the Fm region is the trigger of hypermelanization in internal organs of Silky chickens.
MELANOCYTES are the main cell type, producing pigment that displays body color in birds and mammals. The precursor cells of melanocytes, melanoblasts, derived from the neural crest with other progenitor cells during early embryogenesis (Nordlund et al. 2006). The neural crest cells in the trunk region migrate through either the ventral or dorsolateral route and differentiate according to their presumptive fate (Le Douarin and Kalcheim 2009). The former cells, which migrate through the ventral pathway, become sensory and autonomic neurons, adrenomedullary cells, and Schwann cells (Weston 1963; Le Douarin and Teillet 1974). The latter cells, melanoblasts, migrate at a slightly later stage than the former cells, differentiate into melanocytes, and settle in the integumental basal layer (Erickson and Goins 1995; Le Douarin and Kalcheim 2009).
The Silky chicken (Gallus gallus) is a unique chicken breed with numerous characteristics such as brilliant silky feathers, feathered legs, polydactyly, blue earlobes, etc. In particular, the hyperpigmentation in tissues and organs such as the dermal layer of skin, bone, muscle, pleura, trachea, blood vessels, abdominal lining, and connective tissue (Kuklenski 1915; Hutt 1949) has been noteworthy for breeders and scientists since the 13th century in China (Haw 2006). The huge number of cells containing black melanin particles (melanosomes) in the connective tissue and sheaths of the internal organs is observed in Silky chickens. Therefore Silky chicken meat looks black, which has led to the belief that it contains unknown physiologically useful substance(s) for Chinese cuisine. Many researchers have studied the specific Silky substance(s) but little has been uncovered so far. The origin, history of Fm, other chicken breeds with Fm, and the molecular mechanism that induces the hyperpigmentation in Silky have not yet been completely clarified.
On the pigmentation in dermal tissues of Silky chickens, common melanin was identified as the main pigment substance, and its chemical, physical, and morphological properties were similar to those in other breeds (Muroya et al. 2000; Chen et al. 2008). Cells having melanosomes in internal organs in Silky contain various stages of immature melanosomes in their cell bodies (Reedy et al. 1998; Faraco et al. 2001; Ortolani-Machado et al. 2007, 2009), indicating that melanization occurs inside these cells. Therefore, these cells could be judged as melanocytes. Basically, epidermal melanocytes in humans transfer mature melanosomes to adjacent keratinocytes via melanocyte dendrites (Boissy 2003). However, melanosomes in the dermal skin, eye, inner ear, and leptomeninx in humans and mice are not transferred to the surrounding cells (Okawa et al. 1979; Hori et al. 1982; Boissy and Hornyak 2006). Melanocytes in internal organs in Silky produce stage III melanosomes (intermediate phase of melanosomes’ production with deposit of melanin in matrix protein) and maintain them inside the cells, as occurs in dermal melanocytes in mammals and other fowl (Ortolani-Machado et al. 2009). Thus, there are no distinctive differences in the pigment particles and morphologies of melanosomes or in melanogenesis between dermal melanocytes in Silky chicken and melanocytes in other fowl and mammals.
Silky melanoblasts migrate through an unusual ventral pathway with other neural crest derivatives in addition to the common dorsolateral route and proliferate actively during migration (Hallet and Ferrand 1984; Erickson 1993; Lecoin et al. 1995; Reedy et al. 1998; Muroya et al. 2000; Faraco et al. 2001; Jacobs-Cohen et al. 2002; Le Douarin and Kalcheim 2009). These melanoblasts invade the internal organs and settle among the connective tissues in a similar fashion to fibroblasts. Given the similarity in behavior and localization of these dermal melanoblasts and fibroblasts, Hutt (1949) named this phenotype Fibromelanosis (Fm). Identification of the gene(s) that controls dermal hyperpigmentation in Silky chickens is particularly important to understand the general mechanism on the cell fate determination and migration of pluripotent cells from the neural crest.
Although melanoblasts in mammals and birds are known to migrate mainly via the dorsolateral route from the neural crest, those in lower vertebrates are often observed to travel through the ventral route (Collazo et al. 1993; Raible and Eisen 1994, 1996; Kelsh 2004; Akiyama et al. 2006a; Tomlinson et al. 2009; Reyes et al. 2010). Investigation of Silky chickens could also provide significant clues to recognize the evolutionary divergence of melanoblast migration.
In addition to the Fm locus, Id (the sex-linked inhibitor of dermal melanin) affects hyperpigmentation (Bateson and Punnett 1911; Dunn and Jull 1927). Id is an incomplete dominant sex-linked gene with a role as a modifier for Fm, and combinations of mutants (Id and Fm) and/or wild-type (id+ and fm+) alleles on each locus determine the degree of pigmentation of internal organs in each individual (Stolle 1968). By linkage analyses, Id has been localized on the long arm of chicken chromosome Z (Bitgood 1988; Levin et al. 1993; Dorshorst and Ashwell 2009). Recently, Dorshorst et al. (2010) identified the physical location of Id and also Fm by a genome-wide single nucleotide polymorphism (SNP)-trait association analysis; they demonstrated SNP markers that associated with Id and Fm at 72.3 Mb on chromosome Z and at 10.3–13.1 Mb on chromosome 20, respectively. However, the genomic regions of Id and Fm extend several megabases and still contain a large number of candidate genes.
Since 2006, we have been generating families for chromosome mapping to identify the gene responsible for Fm. Here, we performed linkage mapping analysis by using White Silky and Black Minorca chickens, after evaluating the effects of other loci on Fm. We succeeded in pinpointing the genomic region of Fm. And further, we found noteworthy gene duplication completely linked to Fm. We discuss the correlation between gene duplication and hyperpigmentation in internal organs.
Materials and Methods
Chicken lines
All the chicken lines have been maintained over seven generations as a closed colony and were supplied by Avian Bioscience Research Center, Nagoya University, Nagoya, Japan. White Silky (WS) and Black Minorca (BM-C) were used for Fm mapping. Black Silky (BS), Fayoumi (PNP/DO), and Red Jungle Fowl (RJF) were used as references for the Fm (BS) or fm (wild type) (PNP/DO and RJF) lines (Figure 1). All experiments were performed in accordance with the Nagoya University and Keio University institutional guidelines for animal experiments.
Figure 1 .
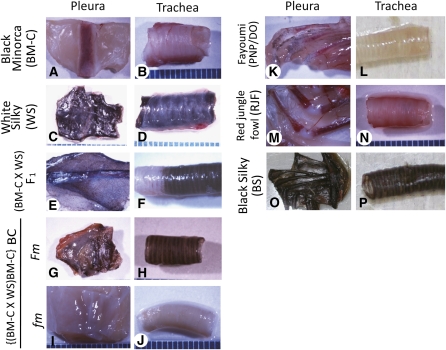
Pigmentation of pleura and trachea in several chicken lines. WS and BS have heavily pigmented internal organs whose colors are clearly different from those in the fm lines (BM-C, PNP/DO, and RJF). F1 between BM-C and WS shows Fm, and BC progeny between the F1 and BM-C are classified into Fm or fm groups. (A and B) BM-C. (C and D) WS. (E and F) F1. (G and H) BC judged as Fm. (I and J) BC judged as fm. (K and L) PNP/DO. (M and N) RJF. (O and P) BS. (A, C, E, G, I, K, M, and O) Pleura. (B, D, F, H, J, L, N, and P) Trachea. (Size) one pitch of the scales: 1 mm.
Identification of the Fm phenotype
We observed pigmentation of pleura, trachea, muscle, bone, and skin dissected out from each chicken at least 2 weeks after hatching (Figure 1); individuals with heavy pigmentation were defined as Fm by visual judgment and stereomicroscopy. Although there was a clear difference in color between the Fm and fm individuals (Figure 1), we confirmed the phenotype quantitatively by measuring the brightness of some internal organs as a Y value with a chronometer (CR-221, Minolta, Tokyo, Japan). The average Y value of each group was calculated using mean values of pleura in individuals. The Y value of the standard white board was 88.7. Y values in the fm lines (BM-C, PNP/DO, and RJF) were clearly different from the Fm lines (WS and BS) (Supporting Information, Figure S1). The Y value in F1 between WS and BM-C (5.8) was close to but significantly higher than those in WS (2.6) and BS (3.3) (P < 0.01, by Student’s t-test) (Figure S1). Y values of Fm and fm individuals in BC from BM-C and the F1 showed major differences: BC with Fm showed 7.5, whereas BC with fm displayed 21.6, which are close to F1 (5.8) and BM-C (22.2), respectively (Figure S1). Finally, Fm or fm was determined by visual judgment of the color of the pleura and trachea. Although the colors of the internal organs in fm lines were apparently bright (Figure 1), a few numbers of pigment cells were often observed by using stereomicroscopy. Because of the fewest visceral pigment cells among investigated lines, BM-C was used for genetic mapping in this study.
Genotyping of DNA markers
DNA was extracted from the blood by using a DNeasy Blood and Tissue kit (Qiagen, Tokyo, Japan) according to the manufacturer’s instructions. Microsatellite markers were amplified by use of a PCR method as follows: 2 min at 95°, followed by 10 cycles of 15 sec at 94°, 30 sec at 55°, 30 sec at 72° and 40 cycles of 15 sec at 94°, 30 sec at 50°, and 30 sec at 72°, with a final elongation step of 5 min at 72°. Length polymorphisms of the PCR products were identified by using 12.5% polyacrylamide gel electrophoresis. PCR for the markers we designed (Table S1) was performed for 2 min at 95°, followed by 10 cycles of 15 sec at 94°, 30 sec at 60°, 45 sec at 72° and 30 cycles of 15 sec at 94°, 30 sec at 55°, and 45 sec at 72°, with a final elongation step of 5 min at 72°. Differences in the PCR products were identified through restriction fragment length polymorphisms (RFLPs) by using 7.5% polyacrylamide gel electrophoresis.
Quantification of gene copy numbers and expression levels
Gene copy numbers were quantified using genomic DNA extracted from blood. The DNA concentrations of all samples were adjusted to a total of 20 ng for each real-time PCR assay. Primers were designed to amplify 89- to 136-bp fragments on each locus (Table S2). SYBR Premix Ex Taq II (Takara Bio, Otsu, Japan) was used according to the manufacturer’s protocol in an Applied Biosystems StepOne Real-Time PCR system (Life Technologies Japan, Tokyo, Japan). The PCR conditions were 1 min at 95°, followed by 40 cycles of 5 sec at 95° and 30 sec at 60°. To confirm that each amplified PCR product was specific to each locus, we determined each melting temperature from dissociation curves. The melting temperature was the defined unique value for each primer set, indicating that the same product was amplified by the same primer set from any DNA sample. The copy number of each locus was determined by use of the comparative Ct method (Livak and Schmittgen 2001; Pfaffl 2001). Ct values for the BM-C line were set to reference point 1, and sample copy numbers were calibrated (as 2–ΔCt) against the Ct value for the AS046 locus and calculated as 2–ΔΔCt.
Expression levels were quantified by using total RNA extracted from whole embryos at stage 18 (Hamburger and Hamilton 1951) with the RNeasy Plus Mini kit (Qiagen). RNA concentrations were determined by using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific) and were adjusted to 10 ng for each real-time assay. Primers were designed to amplify 83- to 120-bp fragments in the exons of each gene (Table S3). A One Step SYBR PrimeScript Plus RT-PCR kit (Takara Bio) was used in accordance with the manufacturer’s protocol in the ABI StepOne Real-Time PCR system. The PCR conditions were 5 min at 42°, 10 sec at 95°, then 40 cycles of 5 sec at 95° and 30 sec at 60°. The melting temperature confirmed that the same product had been amplified from all samples. Ct values from the BM-C line were set to 1 and the relative expression levels (as 2–ΔΔCt) were calculated after calibration (as 2–ΔCt) against the Ct value for the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene. All real-time quantitative PCR experiments were performed four times.
Results
Chromosome mapping of Fibromelanosis
Although the history of chicken breeds is uncertain (Crawford 1990), WS has been thought to originate in China. To identify DNA markers for linkage analysis, it is advantageous to cross between breeds with broadly separated genetic origins. Therefore, we chose BM-C and PNP/DO, which were established in Europe and Egypt, respectively, for mating. First, to understand the modes of inheritance of Fm on their genetic backgrounds, four female BM-C or one female PNP/DO was crossed with a WS male, respectively, then seven to eight F1 females and two to three F1 males from each mating were crossed to obtain F2 offspring. We gained 34 and 11 F1 individuals from BM-C × WS and PNP/DO × WS, respectively. All of these F1 progeny displayed the Fm phenotype. Among the F2 offspring, 71% (N = 17) and 74% (N = 98) showed the Fm phenotype, from incrosses of (BM-C × WS) F1 and (PNP/DO × WS) F1, respectively. Because all F1 individuals exhibited Fm and the ratio of Fm to fm in F2 was almost 3:1, we concluded that the expression of the Fm phenotype was controlled by a single dominant gene that was not affected by other loci among these lines. Concerning the Id locus, these results clearly suggest that both the BM-C and PNP/DO lines were the id+/id+ (wild type) homozygotes. Since the BM-C line showed the least pigmentation in internal organs as described in Materials and Methods, we adopted the BM-C for Fm mapping.
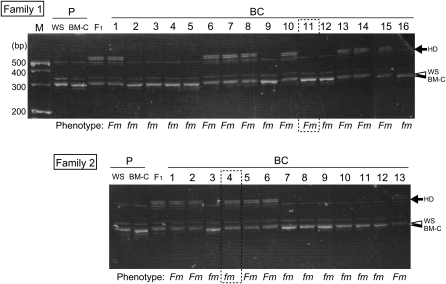
For the mapping, we obtained backcross offspring (BC) from three families (Table 1). Among the BC offspring, 44 (51.8%) showed heavy (Fm) and 41 (48.2%) showed wild-type (fm) pigmentation (Table 1), which corresponded to the expected 1:1 ratio of Fm:fm. We then searched for DNA markers exhibiting length polymorphisms between the WS and BM-C parents of families 1 and 2. We identified 17 polymorphic markers from 46 known microsatellite markers (Takahashi et al. 2005) and genotyped the 29 BC individuals in families 1 and 2. The microsatellite marker ABR0001, which is located on chicken chromosome 20, showed a phenotype-specific polymorphism in BC offspring. Concerning this marker, the WS-specific band was slightly longer than the BM-C–specific band and two F1-specific bands, which could be the result of DNA heteroduplex formation (Ganduly et al. 1993; Hauser et al. 1998), were observed in the F1 individuals (Figure 2). Among the BC progeny, the banding patterns of BM-C and F1 types were distinguishable. The WS- and F1-specific bands were linked to the Fm phenotype in 93% (27/29) individuals (Figure 2), indicating that the position of ABR0001 was close to the Fm locus. To pinpoint the Fm region, we designed new PCR primers by using the draft sequence database for the chicken genome (http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=10804). RFLPs for the PCR products of the parents and the F1 of families 1, 2, and 3 were examined and 10 PCR-RFLP markers were adapted. By using these polymorphic markers, linkage analysis was carried out for the 85 BC progeny.
Table 1. The Fm phenotype in backcross progeny used for Fm mapping.
| Crossing | Phenotype in BC | ||||
|---|---|---|---|---|---|
| Family | ♀ | ♂ | Fm | fm | Total |
| 1 | BM-C | (BM-C × WS) F1 | 9 | 7 | 16 |
| 2 | (WS × BM-C) F1 | BM-C | 5 | 8 | 13 |
| 3 | (WS × BM-C) F1 | BM-C | 30 | 26 | 56 |
| Total | 44 | 41 | 85 | ||
| (51.8%) | (48.2%) | ||||
BC, backcross.
Figure 2 .
An example of linkage analysis using microsatellite markers on the BC progeny. Electrophoretic patterns of ABR0001 PCR products in families 1 and 2 are shown. PCR products in WS (open arrowhead) are slightly longer than those in BM-C (solid arrowhead). Heterozygote-specific heteroduplex bands (HD: arrow) appear in F1 individuals. The WS-specific band and HD cosegregated with Fm in BC individuals except for two individuals (indicated by dotted rectangles).
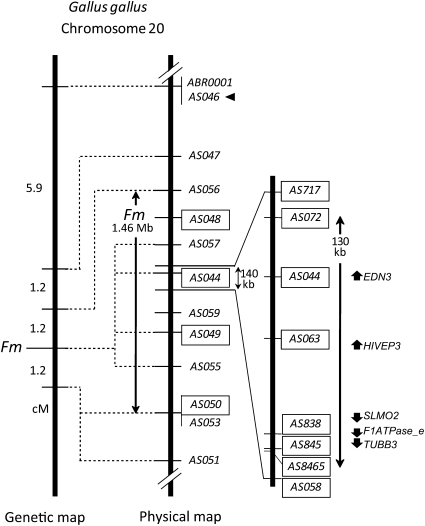
As a result, Fm was mapped to the same position with four DNA markers, AS057, AS044, AS049, and AS055 on chicken chromosome 20 (Figure 3 and Figure S2). Fm and adjacent markers were located in the following order: AS056, Fm, and AS050/AS053/AS051, each separated by a distance of 1.2 cM. On the basis of the chicken sequence database, the position of Fm was 10.2–11.7 Mb on chromosome 20 (Figure 3 and Table S1). Dorshorst et al. (2010) reported the position of Fm as 10.3–13.1 Mb on chromosome 20 by using SNP-trait association analysis with >350 individual backcrossed progeny from F1 females (from a Polish female and a Silky male) and the same Polish male. The Fm region identified in our study is consistent with the Fm position mapped by Dorshorst et al. (2010). Our results thus confirm the position of Fm and narrow down the physical distance of the Fm region to 1.46 Mb from 2.8 Mb.
Figure 3 .
Genetic (left) and physical (right) maps of Fm on chicken chromosome 20. These maps were derived by our study and drawn with a reference of Gallus gallus draft genome sequence data (http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=10804). The 1.46-Mb Fm region and the 130-kb duplicated area are shown by double-headed arrows. Positions of DNA markers used in this study are indicated in the physical map. Details of these markers are shown in Table S1 and Table S2. Loci analyzed by qPCR were indicated by rectangles. A primer set for AS046 (arrowhead) served as a reference for qPCR analyses. Abbreviations of gene names are indicated in Results. Arrows indicate the direction of the genes.
Variations in gene copy number within the Fm region
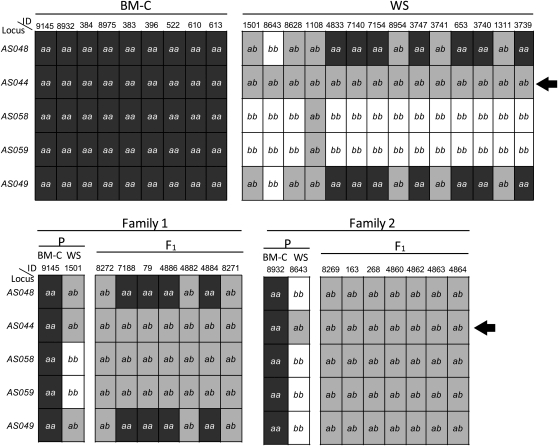
While searching for DNA polymorphisms between BM-C and WS parents, we found a DNA marker linked to Fm, AS044, showing an unusual pattern. Its primer set amplified 1119-bp DNA fragments. All of the PCR-RFLP products in BM-C (n = 9) had a single MboI recognition site, and then we detected two DNA bands on polyacrylamide gel electrophoresis after digestion with it (this pattern is indicated by “aa” in Figure 4). On the other hand, all of the products in WS (n = 14) exhibited a nondigested band together with the same two bands of BM-C, which was considered to be a heterozygous banding pattern (this pattern is indicated by “ab” in Figure 4). In addition, all F1 individuals (n = 14) from families 1 and 2 showed the same heterozygous pattern as that of WS. When we examined other DNA markers, the allelic inheritance modes of the PCR-RFLPs followed Mendel’s law (Figure 4). These results suggest that the AS044 locus is duplicated in the WS line and that a sequence polymorphism exists between the original and duplicated DNA sequences.
Figure 4 .
Genotyping data for five Fm-linked DNA markers in BM-C (n = 9), WS (n = 14), and F1 (n = 14) between BM-C and WS in families 1 and 2. A haplotype pattern of PCR-RFLP is indicated as a or b. Individuals that showed the a or b band pattern are described as aa or bb, respectively, and individuals that displayed the a plus b band pattern are shown as ab. On the AS044 locus (arrows), all WS and F1 individuals displayed the ab band pattern. ID, identification number. Details of these loci are shown in Table S1.
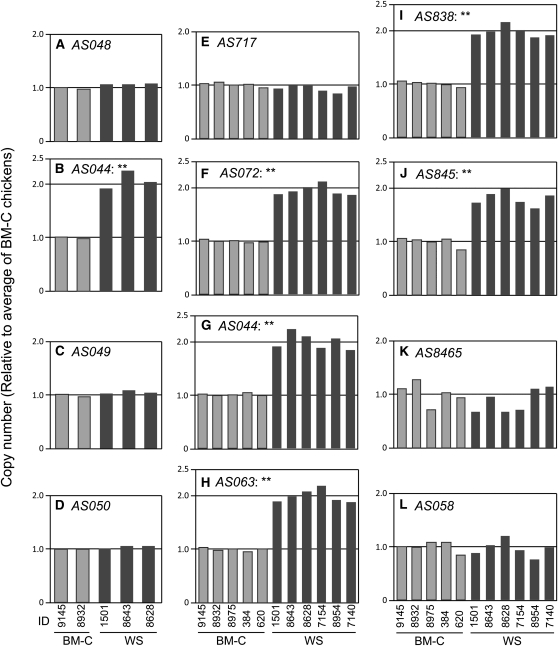
To reveal the gene duplication, we analyzed the genomic copy number of several loci by using quantitative real-time PCR. Initially, we analyzed the genomic copy numbers of four loci, AS048, AS044, AS049, and AS050 in two BM-C and three WS individuals (Figures 3 and 5, details of primers are indicated in Table S2). Gene copy numbers of three of these loci (AS048, AS049, and AS050) were the same for BM-C and WS chickens (Figure 5, A, C, and D). However, the relative copy number in WS of the AS044 locus was twice that in BM-C (Figure 5B). Because there were no differences in copy number among individuals in the same line, this copy number duplication was considered to be WS specific. To further clarify this gene duplication, we analyzed eight loci including the AS044 locus in the 140-kb area of the Fm region and increased sampling to five and six individuals of BM-C and WS, respectively (Figures 3 and 5). For five loci from AS072 to AS845, the genomic copy number in WS was almost twofold of that in BM-C (Figure 5 F–J), whereas those for the other three loci, AS717, AS8465, and AS058, in WS were similar to BM-C individuals (Figure 5, E, K, and L). For all loci except AS8465, there were no considerable differences among individuals in the same line. Although a small variation was observed on the AS8465 locus, it was not strain specific. These results clearly demonstrate that the genome in the WS line has a duplicated part in the Fm region and that this duplicated area was within 130 kb from the border between AS717 and AS072 to that between AS845 and AS8465 (Figure 3).
Figure 5 .
Quantitative PCR analysis of genomic copy numbers in the BM-C and WS lines. Copy numbers in BM-C (light-shaded columns) and WS (dark-shaded columns) individuals, relative to that for the average of BM-C chickens, were calculated by use of the comparative Ct method. Data were normalized to the reference locus (AS046). Double asterisks indicate a statistically significant difference between BM-C and WS lines (P < 0.01, by Student’s t-test). Locations of analyzed loci are represented in Figure 3.
Correlation between gene duplication and Fm
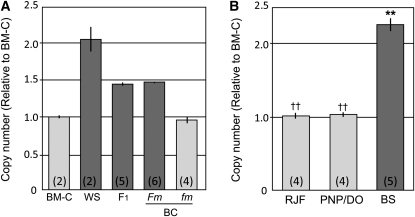
To determine whether both copies of the duplicated area were located in the Fm-linked region, we investigated the linkage of the copy number of AS044 to the Fm phenotype by using families 1 and 2. Two or three F1 individuals, as well as three and two with Fm and fm phenotypes in BC, respectively, were randomly selected from each family and analyzed together with the parent chickens. The copy number in the F1 was 1.5 times that of BM-C, which was the middle value between the parents (Figure 6A). The copy number in BC with Fm was also 1.5 times, whereas that for BC with fm was almost equal to that in BM-C (Figure 6A). There were no differences among individuals in the same group and between families. These results indicate that the gene duplication is linked to the Fm phenotype and that WS chickens have two, namely an original and a duplicated, segments in the Fm region on each chromosome 20, whereas BM-C chickens have only the original segment on this chromosome.
Figure 6 .
Quantitative PCR analyses of genomic copy numbers of the AS044 locus in the families used for the linkage mapping and other breeds. Copy numbers relative to that for BM-C were calculated by using the comparative Ct method. Data were normalized to the reference locus (AS046). Light-shaded and dark-shaded columns indicate the fm and Fm phenotypes, respectively. Sample numbers used for the analysis are indicated in parentheses. The gene duplication was linked to the Fm phenotype in BC progeny in families 1 and 2 (A). Chicken breeds displaying fm (RJF and PNP/DO) and Fm (BS) demonstrated equal and almost twofold of the copy number in BM-C, respectively (B). Double asterisks and daggers indicate a statistically significant difference for BM-C and WS lines, respectively (P < 0.01, by Student’s t-test). Bar, ±SE.
To elucidate the correlation between the Fm phenotype and copy number variation, we examined the copy numbers of the AS044 locus in other chicken lines that display the Fm or fm phenotype. In fm lines (RJF and PNP/DO), the copy numbers were equal to that in BM-C, whereas the value in BS (Fm) was almost twice that in BM-C (Figure 6B). These data show that: (1) part of the Fm-linked region in chromosome 20 is duplicated in Silky lines, and (2) this gene duplication is specific to Fm chickens; fm chickens have only one copy on chromosome 20. These results strongly suggest that gene duplication is responsible for the Fm phenotype.
We maintain another chicken line that has heavy melanization in its internal organs, similar to that in Silky chickens. This line originated from two Indonesian chickens, Ayam cemani and Ayam Arab. The internal organs of Ayam cemani were black, whereas those of Ayam Arab showed the wild-type phenotype. We crossed an Ayam cemani female with an Ayam Arab male and all of the F1 progeny displayed black internal organs, indicating that the hyperpigmentation in Ayam cemani was a dominant trait like Fm in Silky. This line has been maintained by incrosses among these siblings, and the progeny included two types of individuals of which internal organs showed dark and wild-type color. By using one generation (N = 11) in this line, we examined the copy number of AS044. The copy number of individuals with dark color internal organs (N = 6) was almost 1.5 times that in the siblings with wild-type color (N = 5), and there was a statistically significant difference between them (P < 0.01, by Student’s t-test). Namely, the copy number of siblings in the Ayam cemani line is also correlated with the color of internal organs, suggesting that the original Ayam cemani chicken, which displays hyberpigmentation in internal organs, also had a duplicated segment containing AS044.
Expression levels of genes located on the duplicated segment
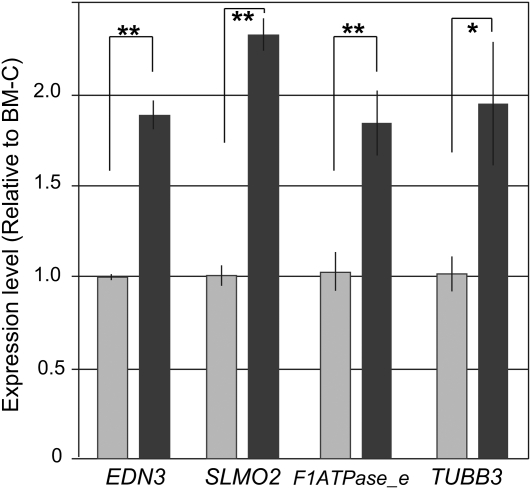
In the 130-kb area duplicated in the WS genome, five genes, which are homologous to endothelin 3 (EDN3), HIVEP1, slowmo homolog2 (SLMO2), H+ transporting F1 ATP synthase epsilon subunit (F1ATPase-e), and tubulin beta 3 (TUBB3), are annotated on the basis of information in the ENTREZ Genome Project database (Gallus gallus Build 2.1; http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=10804) (Figure 3). In chicken embryos, melanoblasts first begin to migrate from the neural crest at stage 18 and enter the dorsolateral path at around stage 20. Only a few of them generally enter the ventral area after that. In Silky embryos, a greater number of the melanoblasts are observed in the ventral area at stage 22 than those in other fowls (Reedy et al. 1998; Faraco et al. 2001). To clarify whether the five genes are expressed in chicken embryos during melanoblast migration, we analyzed the mRNA expression of these genes by using RNA extracted from whole embryos at stage 18. Four of the five genes (not HIVEP1) were detected by using RT-PCR (data not shown). We then quantified the expression levels of these four genes and compared them between BM-C and WS embryos. For all four genes, the mRNA levels in the WS embryos were significantly higher than those in the BM-C embryos; that of EDN3, SLMO2, F1ATPase-e, and TUBB3 was 1.9, 2.4, 1.9, and 1.9 times higher, respectively (Figure 7). These results correspond with the copy number duplication in the WS line and suggest that the gene duplication caused the hyperexpression of these genes.
Figure 7 .
Quantitative PCR analysis of expression levels of genes located in the duplicated 130-kb area in whole embryos at stage 18 in BM-C (n = 5, light-shaded column) and WS (n = 7, dark-shaded column). Gene expression levels in WS relative to those in BM-C were obtained by using the comparative Ct method. Data were normalized to a reference gene (GAPDH; glyceraldehyde-3-phosphate dehydrogenase). The gene positions are indicated in Figure 3. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01; by Student’s t-test). Bar, ±SE.
Discussion
Our findings in the present study confirm that the Fm phenotype in Silky chickens is mainly controlled by a single locus. Other unique characteristics, silky feathers, feathered legs, polydactyly, a mulberry crest, and hair bulb in head, were not linked to the Fm, demonstrating that these are controlled by other loci. The Fm region was within 1.46 Mb on chicken chromosome 20 and further narrowed relative to a previous report by Dorshorst et al. (2010). Furthermore, we found gene duplication in the Fm region of the genome in White and Black Silky chickens, and this duplication was not observed in other chicken breeds with wild-type pigmentation in their dermal tissues. We also detected a linkage between hypermelanization and gene duplication in independent chicken lines, that is, White Silky and Ayam cemani. These results strongly suggest that the gene duplication affects the hypermelanization in dermal tissues.
Chromosomal segmental copy number variation (CNV) has been recently recognized as a very important source of genetic variability (Jiang et al. 2004; Redon et al. 2006; McCarroll et al. 2008; Conrad et al. 2010). Some CNV loci contain genes or conserved regulatory elements that affect mRNA expression levels (Stranger et al. 2007). Furthermore, recent studies have found CNVs related to various human diseases (Wain et al. 2009; Zhang et al. 2009; Stankiewicz and Lupski 2010). CNVs are also observed in Aves both in inter- and intraspecies (Griffin et al. 2008; Skinner et al. 2009; Völker et al. 2010; Wang et al. 2010). In addition, an association between CNVs and a specific phenotype has been documented (Wright et al. 2009). Intraspecific CNVs for the chicken genome were analyzed by using NimbleGen whole genome tiling arrays with 385,000 probes; the mean probe spacing was ∼2.6 kb (Wang et al. 2010). However, the 130-kb CNV in this study was not found by them; we are, therefore, the first to report this CNV between Silky fowl and other chicken breeds. Since Wang et al. (2010) used three fm lines (broilers, Leghorns, and Rhode Island Reds), we believe that none of these lines has a duplicated copy of the region. This may explain why the CNV was not detected by their whole chicken genome assay. Chickens represent a very important farm animal species that has also long served as a model for biological and biomedical research. Further studies will disclose the correlation between CNVs and various distinctive traits that are segregated and established as chicken breeds.
In the 130-kb duplicated area, five genes were annotated on the basis of information in the ENTREZ Genome Project database. mRNAs were expressed from four of these five genes in whole embryos at stage 18 just before initiation of melanoblast migration (Reedy et al. 1998; Faraco et al. 2001). The expression levels of all four of these genes in Silky chickens were 1.9 to 2.4 times those in BM-C, which concurred with the gene copy numbers. On the other hand, distribution of the EDN3 mRNA detected by in situ hybridization studies was not significantly different between Silky and other fm fowl embryos (data not shown). These results suggest that the mRNAs are transcribed from both the original gene and the duplicated copy and that there are no obvious differences in cis-regulatory elements between the two sequences of these genes. Although all F1 progeny between BM-C and WS displayed the Fm phenotype, the degree of pigmentation in their internal tissues was significantly lighter than that in WS (Figure S1). This observation indicates that the hyperpigmentation in the Fm/Fm homozygote is more severe than that in the Fm/fm+ heterozygote. Therefore, the Fm phenotype can be considered as a semidominant rather than a dominant trait. It corresponds well to the copy numbers of the duplicated area (Figure 6A). From these results, we propose the following hypothesis: gene duplication leads to high levels of mRNA expression, which, in turn, triggers hypermelanization in internal organs. The degree of pigmentation could correlate with the mRNA expression level.
Of the four genes that displayed high mRNA expression, we propose that EDN3, in particular, is a candidate gene for Fm. Vasoactive endothelin (EDN) was first described by Yanagisawa et al. (1988). The endothelin family comprises three 21-amino acid peptides, EDN 1, 2, and 3, that are highly conserved. In mammals, two endothelin receptors (EDNRs), EDNRA and EDNRB, which belong to a G protein-coupled heptahelical superfamily (Arai et al. 1990; Sakurai et al. 1990, 1992; Kusserow and Unger 2004), have been identified. EDNRA has high affinities for EDN1 and EDN2 and a significantly lower affinity for EDN3 (Arai et al. 1990), whereas EDNRB exhibits similar affinities for all three EDNs (Sakurai et al. 1990, 1992). EDN3 and EDNRB are both allelic to the spontaneous mouse mutations that occur at the lethal spotting (ls) and piebald lethal (sl) loci, respectively. Recessive mutants of these loci yield similar phenotypes that consist of differing degrees of hypopigmentation and aganglionic megacolon (Baynash et al. 1994; Hosoda et al. 1994). The hypopigmented phenotype has been attributed to a decrease in the melanoblast population and to abnormal cell migration (Lee et al. 2003; Pavan and Tilghman 1994). A paralogue of EDNRB (designated EDNRB2), found to be specific to the melanocytic lineage, was cloned in quail by Lecoin et al. (1998). A mutant in quail, which had an amino acid substitution and reduced gene expression in the ENDRB2 gene, displayed white plumage with wild-type colored spots (Miwa et al. 2007). In the mutant embryos, pigment production in the integument and feather bud was strongly suppressed from the early developmental stage, and few melanoblasts survived (Akiyama et al. 2006b). These analyses clearly demonstrate that the signal transduction system of EDN3–EDNRB in mammals and EDN3–EDNRB2 in birds has a crucial role in melanoblast/melanocyte development from neural crest cells. Of the other three genes that were highly expressed in Silky embryos (Figure 7), H+ transporting F1 ATP synthase epsilon subunit may relate with the phenotype because F1F0 mitochondrial ATP synthase has been reported as a target for modulating pigmentation of melanocytes (Jung et al. 2005). More studies are necessary to determine whether these three genes are involved in the melanoblasts/melanocytes’ development.
In Silky embryos, two distinctive events occur during melanoblast development: accelerated proliferation and unusual ventral migration. Numerous in vitro studies using quail and mouse embryos have reported that EDN3 affects the melanocyte lineage population by increasing their number in a dose-dependent manner (Lahav et al. 1996, 1998; Reid et al. 1996; Opdecamp et al. 1998; Dupin et al. 2000). In addition, exogenous overexpression of EDN3 driven by keratin 5 in transgenic mouse embryos induced proliferation of melanocyte precursors and led to hyperpigmentation on most areas of their skin (Garcia et al. 2008). Here, the high-level expression of EDN3 mRNA in Silky chickens was detected at stage 18, and we obtained the same result at stages 15 and 24 (data not shown). These data suggest that Silky embryos are exposed to high doses of EDN3 before and during differentiation of melanoblasts from neural crest cells, and that abundant EDN3 in vivo could induce the accelerated proliferation of melanocyte precursors. Experiments with grafts between embryos of Silky and other fowl (Hallet and Ferrand 1984; Ferrand and L’Hermite 1985) and the culture of neural crest cells isolated from quail embryos in medium containing embryonic extract from Silky or other fowl (Lecoin et al. 1994), suggest that Silky embryos contain a growth factor(s) for melanocyte proliferation and that the Fm phenotype is not attributable to the melanocyte lineage but rather to other cell types in the melanoblast environment. EDN3, which encodes a ligand of EDNRB2, is expressed in the ectoderm and in gut mesenchyme (Nataf et al. 1998), whereas EDNRB2 is expressed throughout the melanocyte lineage (Lecoin et al. 1998). From these data and our present results, we suggest that a mitogen contained in Silky embryos for the melanocyte lineage is abundant EDN3 due to the gene duplication.
It remains unclear whether the unusual ventral migration of melanoblasts in Silky embryos is a result of excess EDN3. In Xenopus embryos, many melanoblasts have been observed migrating through the ventral pathway (Collazo et al. 1993). Kawasaki-Nishihara et al. (2011), suggest that EDN3–EDNRB2 signaling in Xenopus embryos is essential for normal migration of melanoblasts by in vivo experiments of EDNRB2 overexpression and inhibition of EDN3 expression. In the case of Aves, EDNRB2 is thought to be important for melanoblast migration toward the usual “dorsolateral” pathway (Pla et al. 2005; Harris et al. 2008). On the other hand, Aoki et al. (2009) demonstrated that noncutaneous and dermal melanocytes are more sensitive to EDN3 for growth and differentiation compared with epidermal melanocytes. In Silky fowl, excess EDN3 may affect only the proliferation of the dermal melanocyte cell lineage during the early differentiating stage. Thereafter, these proliferated cells would disperse to other accessible sites. It may result in the abnormal migration of melanoblasts and the distribution of melanocytes in internal organs. Excess EDN3 may actually induce the sequential expression of other genes involved in signal transduction or of extracellular matrix proteins, which could lead to melanoblast proliferation and ventral migration. Even so, the excess production of EDN3 in Silky fowl as a result of gene duplication could be the first trigger for hypermelanization.
Our results and hypothesis seem appropriate to explain the hyperpigmentation in Silky chickens. Silky hyperpigmentation is a valuable model to study one of the basic important biological themes, that is, cell migration and fate determination of pluripotent neural crest cells. Additional studies are necessary to fully understand this phenomenon, some of which are now in progress.
Acknowledgments
The authors deeply thank Katsutoshi Kino, Koichiro Hashimoto, and Hiroshi Ogawa for first supplying the chickens in pilot experiments. We are also grateful to Takayuki Teramoto, Atsushi Kurabayashi, Mitsuru Miwa, and Susanne Kerje for useful suggestions regarding the genetic mapping. We gratefully acknowledge the technical support of Tsuneo Suzuki with respect to use of the chronometer. This work was supported in part by Keio University funds for T.A. and A.S.
Footnotes
Communicating editor: L. D. Siracusa
Literature Cited
- Akiyama T., Matsumoto J., Kitamura K., 2006a. Immunocytochemical studies on the differentiation of melanophores and its relation to migratory behaviors in goldfish Carassius auratus. Hiyoshi Rev. Nat. Sci. Keio Univ. 39: 1–20 [Google Scholar]
- Akiyama T., Miwa M., Inoue-Murayama M., Mizutani M., Kayashima Y., et al. , 2006b. Endothelin Receptor B2 Mutated Japanese quail with panda plumage color shows hypopigmentation during development. Pigment Cell Res. 19: 516 [Google Scholar]
- Aoki H., Yamada Y., Kunisada T., 2009. Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of noncutaneous and dermal vs. epidermal melanocytes. Development 136: 2511–2521 [DOI] [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S., 1990. Cloning and expression of cDNA encoding an endothelin receptor. Nature 348: 730–732 [DOI] [PubMed] [Google Scholar]
- Bateson W., Punnett R., 1911. The inheritance of the peculiar pigmentation of the silky fowl. J. Genet. 1: 185–203 [Google Scholar]
- Baynash A. G., Hosoda K., Giaid A., Richardson J. A., Emoto N., et al. , 1994. Interaction of Endothelin-3 with Endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79: 1277–1285 [DOI] [PubMed] [Google Scholar]
- Bitgood J. J., 1988. Linear relationship of the loci for barring, dermal melanin inhibitor, and recessive white skin on the chicken Z chromosome. Poult. Sci. 67: 530–533 [DOI] [PubMed] [Google Scholar]
- Boissy R. E., 2003. Melanosome transfer to and translocation in the keratinocyte. Exp. Dermatol. 12: 5–12 [DOI] [PubMed] [Google Scholar]
- Boissy R. E., Hornyak T. J., 2006. Extracutaneous melanocytes, pp. 91–107 in The Pigmentary System: Physiology and Pathophysiology, Ed. 2, edited by J. J. Nordlund, R. E. Boissy, V. J. Hearing, R. A. King, W. S. Oetting, and J. P. Ortonne. Oxford University Press, New York [Google Scholar]
- Chen S., Jiang B., Zheng J., Xu G., Li J., et al. , 2008. Isolation and characterization of natural melanin derived from silky fowl(Gallus gallus domesticus Brisson). Food Chem. 111: 745–749 [Google Scholar]
- Collazo A., Bronner-Fraser M., Fraser S. E., 1993. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development 118: 363–376 [DOI] [PubMed] [Google Scholar]
- Conrad D. F., Pinto D., Redon R., Feuk L., Gokcumen O., et al. , 2010. Origins and functional impact of copy number variation in the human genome. Nature 464: 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. D., 1990. Poultry Breeding and Genetics. Elsevier Science Publishers, Amsterdam, The Netherlands [Google Scholar]
- Dorshorst B. J., Ashwell C. M., 2009. Genetic mapping of the sex-linked barring gene in the chicken. Poult. Sci. 88: 1811–1817 [DOI] [PubMed] [Google Scholar]
- Dorshorst B., Okimoto R., Ashwell C., 2010. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the Silkie chicken. J. Hered. 101: 339–350 [DOI] [PubMed] [Google Scholar]
- Dunn L., Jull M., 1927. On the inheritance of some characters on the silky fowl. J. Genet. 19: 27–63 [Google Scholar]
- Dupin E., Glavieux C., Vaigot P., Le Douarin N. M., 2000. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc. Natl. Acad. Sci. USA 97: 7882–7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., 1993. From the crest to the periphery: control of pigment cell migration and lineage segregation. Pigment Cell Res. 6: 336–347 [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Goins T. L., 1995. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development 121: 915–924 [DOI] [PubMed] [Google Scholar]
- Faraco C. D., Vaz S. A., Pastor M. V., Erickson C. A., 2001. Hyperpigmentation in the Silkie fowl correlates with abnormal migration of fate-restricted melanoblasts and loss of environmental barrier molecules. Dev. Dyn. 220: 212–225 [DOI] [PubMed] [Google Scholar]
- Ferrand R., L'Hermite A., 1985. Experimental analysis of the extensive pigmentation in the Silkie fowl embryo: evidence for an environmental regulatory process. Experientia 41: 512–514 [DOI] [PubMed] [Google Scholar]
- Ganduly A., Rock M. J., Prockop D. J., 1993. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc. Natl. Acad. Sci. USA 90: 10325–10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R. J., Ittah A., Mirabal S., Figueroa J., Lopez L., et al. , 2008. Endothelin 3 induces skin pigmentation in a keratin-driven inducible mouse model. J. Invest. Dermatol. 128: 131–142 [DOI] [PubMed] [Google Scholar]
- Griffin D. K., Robertson L. B., Tempest H. G., Vignal A., Fillon V., et al. , 2008. Whole genome comparative studies between chicken and turkey and their implications for avian genome evolution. BMC Genomics 9: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallet M. M., Ferrand R., 1984. Quail melanoblast migration in two breeds of fowl and in their hybrids: evidence for a dominant genic control of the mesodermal pigment cell pattern through the tissue environment. J. Exp. Zool. 230: 229–238 [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L., 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88: 49–92 [PubMed] [Google Scholar]
- Harris M. L., Hall R., Erickson C. A., 2008. Directing pathfinding along the dorsolateral path – the role of EDNRB2 and EphB2 in overcoming inhibition. Development 135: 4113–4122 [DOI] [PubMed] [Google Scholar]
- Hauser M. T., Adhami F., Dorner M., Fuchs E., Glossl J., 1998. Generation of co-dominant PCR-based markers by duplex analysis on high resolution gels. Plant J. 16: 117–125 [DOI] [PubMed] [Google Scholar]
- Haw S. G., 2006. Marco Polo’s China: a Venetian in the realm of Khubilai Khan. Routledge studies in the early history of Asia. Ed. 3. Routledge, London, [Google Scholar]
- Hori Y., Ohara Y., Niimura M., Kukita A., 1982. Electron microscopy, ultrastructural observations of the extracellular sheath of dermal melanocytes in the nevus of Ota. Am. J. Dermatophathol. 4: 245–251 [PubMed] [Google Scholar]
- Hosoda K., Hammer R. E., Richardson J. A., Baynash A. G., Cheung J. C., et al. , 1994. Targeted and natural (Piebald-Lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79: 1267–1276 [DOI] [PubMed] [Google Scholar]
- Hutt F. B., 1949. Genetics of the Fowl. McGraw-Hill, New York [Google Scholar]
- Jacobs-Cohen R. J., Wade P. R., Gershon M. D., 2002. Suppression of the melanogenic potential of migrating neural crest-derived cells by the branchial arches. Anat. Rec. 268: 16–26 [DOI] [PubMed] [Google Scholar]
- Jiang L. J., Mao J. H., Balmain A., Peterson L., Harris C., et al. , 2004. Genomic segmental polymorphisms in inbred mouse strains. Nat. Genet. 36: 952–954 [DOI] [PubMed] [Google Scholar]
- Jung D., Williams D., Khersonsky S. M., Kang T., Heidary N., et al. , 2005. Identification of the F1F0 mitochondrial ATPase as a target for modulating skin pigmentation by screening a tagged triazine library in zebrafish. Mol. Biosyst. 1: 85–92 [Google Scholar]
- Kawasaki-Nishihara A., Nishihara D., Nakamura H., Yamamoto H., 2011. ET3/Ednrb2 signaling is critically involved in regulating melanophore migration in Xenopus. Dev. Dyn. 240: 1454–1466 [DOI] [PubMed] [Google Scholar]
- Kelsh R. N., 2004. Genetics and evolution of pigment patterns in fish. Pigment Cell Res. 17: 326–336 [DOI] [PubMed] [Google Scholar]
- Kuklenski J., 1915. Über das vorkommen und die verteilung des pigmentes in den organen und geweben bei japanischen seidenhühnern. (Over occurrence and the distribution of the pigment in the organs and tissues of Japanese Silky chickens.) Arch. Micro. Anat. Entwickl. 87: 1–37 [Google Scholar]
- Kusserow H., Unger T., 2004. Vasoactive peptides, their receptors and drug development. Basic Clin. Pharmacol. Toxicol. 94: 5–12 [PubMed] [Google Scholar]
- Lahav R., Ziller C., Dupin E., Le Douarin N. M., 1996. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc. Natl. Acad. Sci. USA 93: 3892–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R., Dupin E., Lecoin L., Glavieux C., Champeval D., et al. , 1998. Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc. Natl. Acad. Sci. USA 95: 14214–14219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoin L., Mercier P., Le Douarin N. M., 1994. Growth of neural crest cells in vitro is enhanced by extracts from silky fowl embryonic tissues. Pigment Cell Res. 7: 210–216 [DOI] [PubMed] [Google Scholar]
- Lecoin L., Lahav R., Martin F. H., Teillet M. A., Le Douarin N. M., 1995. Steel and c-kit in the development of avian melanocytes: a study of normally pigmented birds and of the hyperpigmented mutant silky fowl. Dev. Dyn. 203: 106–118 [DOI] [PubMed] [Google Scholar]
- Lecoin L., Sakurai T., Ngo M., Abe Y., Yanagisawa M., et al. , 1998. Cloning and characterization of a novel endothelin receptor subtype in the avian class. Proc. Natl. Acad. Sci. USA 95: 3024–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Kalcheim C., 2009. The Neural Crest. Cambridge University Press, Cambridge [Google Scholar]
- Le Douarin N. M., Teillet M. A., 1974. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 41: 162–184 [DOI] [PubMed] [Google Scholar]
- Lee H., Levorse J. M., Shin M. K., 2003. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev. Biol. 259: 162–175 [DOI] [PubMed] [Google Scholar]
- Levin I., Crittenden L. B., Dodgson J. B., 1993. Genetic map of the chicken Z chromosome using random amplified polymorphic DNA (RAPD) markers. Genomics 16: 224–230 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- McCarroll S. A., Kuruvilla F. G., Korn J. M., Cawley S., Nemesh J., et al. , 2008. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat. Genet. 40: 1166–1174 [DOI] [PubMed] [Google Scholar]
- Miwa M., Inoue-Murayama M., Aoki H., Kunisada T., Hiragaki T., et al. , 2007. Endothelin receptor B2 (EDNRB2) is associated with the panda plumage colour mutation in Japanese quail. Anim. Genet. 38: 103–108 [DOI] [PubMed] [Google Scholar]
- Muroya S., Tanabe R., Nakajima I., Chikuni K., 2000. Molecular characteristics and site specific distribution of the pigment of the Silky fowl. J. Vet. Med. Sci. 62: 391–395 [DOI] [PubMed] [Google Scholar]
- Nataf V., Amemiya A., Yanagisawa M., Le Douarin N. M., 1998. The expression pattern of endothelin 3 in the avian embryo. Mech. Dev. 73: 217–220 [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Boissy R. E., Hearing V. J., King R. A., Oetting W. S., et al. , 2006. The Pigmentary System. Blackwell Publishing, Oxford [Google Scholar]
- Okawa Y., Yokota R., Yamauchi A., 1979. On the extracellular sheath of dermal melanocytes in nevus fuscoceruleus acromiodeltoideus (Ito) and Mongolian spot. An ultrastructural study. J. Invest. Dermatol. 73: 224–230 [DOI] [PubMed] [Google Scholar]
- Opdecamp K., Kos L., Arnheiter H., Pavan W. J., 1998. Endothelin signalling in the development of neural crest-derived melanocytes. Biochem. Cell Biol. 76: 1093–1099 [PubMed] [Google Scholar]
- Ortolani-Machado C., De Freitas P., Borges M. E., Faraco C., 2007. Special features of dermal melanocytes in white silky chicken embryos. Anat. Rec. 291: 55–64 [DOI] [PubMed] [Google Scholar]
- Ortolani-Machado C. F., Freitas P. F., Faraco C. D., 2009. Melanogenesis in dermal melanocytes of Japanese Silky chicken embryos. Tissue Cell 41: 239–248 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Tilghman S. M., 1994. Piebald lethal (sl) acts early to disrupt the development of neural crest-derived melanocytes. Proc. Natl. Acad. Sci. USA 91: 7159–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla P., Alberti C., Solov’eva O., Pasdar M., Kunisada T., et al. , 2005. Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation. Pigment Cell Res. 18: 181–187 [DOI] [PubMed] [Google Scholar]
- Raible D. W., Eisen J. S., 1994. Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development 120: 495–503 [DOI] [PubMed] [Google Scholar]
- Raible D. W., Eisen J. S., 1996. Regulative interactions in Zebrafish neural crest. Development 122: 501–507 [DOI] [PubMed] [Google Scholar]
- Redon R., Ishikawa S., Fitch K. R., Feuk L., Perry G. H., et al. , 2006. Global variation in copy number in the human genome. Nature 444: 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy M. V., Faraco C. D., Erickson C. A., 1998. Specification and migration of melanoblasts at the vagal level and in hyperpigmented Silkie chickens. Dev. Dyn. 213: 476–485 [DOI] [PubMed] [Google Scholar]
- Reid K., Turnley A. M., Maxwell G. D., Kurihara Y., Kurihara H., et al. , 1996. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development 122: 3911–3919 [DOI] [PubMed] [Google Scholar]
- Reyes M., Zandberg K., Desmawati I., De Bellard M. E., 2010. Emergence and migration of trunk neural crest cells in a snake, the California Kingsnake (Lampropeltis getula californiae). BMC Dev. Biol. 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., et al. , 1990. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348: 732–735 [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Masaki T., 1992. Molecular characterization of endothelin receptors. Trends Pharmacol. Sci. 13: 103–108 [DOI] [PubMed] [Google Scholar]
- Skinner B. M., Robertson L. B., Tempest H. G., Langley E. J., Ioannou D., et al. , 2009. Comparative genomics in chicken and Pekin duck using FISH mapping and microarray analysis. BMC Genomics 10: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz P., Lupski J. R., 2010. Structural variation in the human genome and its role in disease. Annu. Rev. Med. 61: 437–455 [DOI] [PubMed] [Google Scholar]
- Stranger B. E., Forrest M. S., Dunning M., Ingle C. E., Beazley C., et al. , 2007. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315: 848–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolle I., 1968. Vergleichende untersuchungen über die pigmentierung des seidenhuhns, des Italienerhuhns und ihrer bastarde. (Analysis comparing pigmentation of Silkies, Brown Leghorns and their hybrids.) Wilhelm Roux' Archiv. 160: 30–48 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Tsudzuki M., Sasaki O., Niikura J., Inoue-Murayama M., et al. , 2005. A chicken linkage map based on microsatellite markers genotyped on a Japanese large game and white leghorn cross. Anim. Genet. 36: 463–467 [DOI] [PubMed] [Google Scholar]
- Tomlinson M. L., Guan P., Morris R. J., Fidock M. D., Rejzek M., et al. , 2009. A Chemical genomic approach identifies matrix metalloproteinases as playing an essential and specific role in Xenopus melanophore migration. Chem. Biol. 16: 93–104 [DOI] [PubMed] [Google Scholar]
- Völker M., Backström N., Skinner B. M., Langley E. J., Bunzey S. K., et al. , 2010. Copy number variation, chromosome rearrangement, and their association with recombination during avian evolution. Genome Res. 20: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain L. V., Armour J. A. L., Tobin M. D., 2009. Genomic copy number variation, human health, and disease. Lancet 374: 340–350 [DOI] [PubMed] [Google Scholar]
- Wang X., Nahashon S., Feaster T. K., Bohannon-Stewart A., Adefope N., 2010. An initial map of chromosomal segmental copy number variations in the chicken. BMC Genomics 11: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston J. A., 1963. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 6: 279–310 [DOI] [PubMed] [Google Scholar]
- Wright D., Boije H., Meadows J. R. S., Bed’hom B., Gourichon D., et al. , 2009. Copy number variation in intron 1 of SOX5 causes the pea-comb phenotype in chickens. PLoS Genet. 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., et al. , 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415 [DOI] [PubMed] [Google Scholar]
- Zhang F., Gu W. L., Hurles M. E., Lupski J. R., 2009. Copy number variation in human health, disease, and evolution. Annu. Rev. Genomics Hum. Genet. 10: 451–481 [DOI] [PMC free article] [PubMed] [Google Scholar]