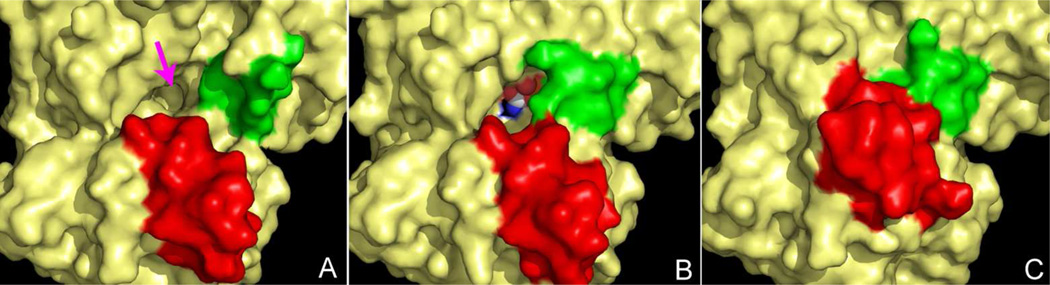
FIGURE 13.
Surface of ATCase near the active site detailing the 80's loop (green) and the 240's loop (red) during catalysis. (A) Conformation of the enzyme before substrates are bound; the approximate position of the active site is indicated by the magenta arrow. (B) Conformation of the enzyme after the binding of CP, which is shown in the active site as a CPK model (white carbon). The 80's loop (green) rearranges to help create the binding site for aspartate. (C) Upon the binding of aspartate the 240's loop (red) undergoes a dramatic conformational change, forcing the substrates toward each other. Solvent is not accessible to the active site in this conformation.