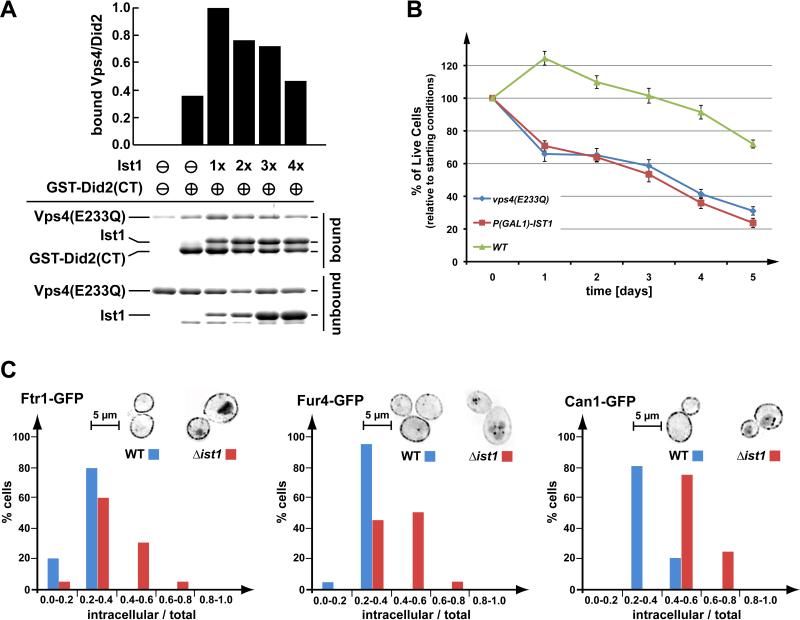
Figure 3.
Regulation of Vps4 by Ist1. (A) In vitro binding studies using recombinant Vps4(E233Q), Ist1 and GST-Did2(CT) (amino acids 113-204). GST-Did2(CT) was immobilized on GSH-sepharose and an approximately equimolar amount of Vps4(E233Q) was added in the presence of ATP. The Ist1 concentrations were varied from zero to approximately four times the molar amount of Vps4(E233Q). Bound and unbound fractions were analyzed by SDS-PAGE and coomassie staining, and the amount of bound Vps4(E233Q) was determined by densitometric scanning. The obtained values were normalized according to the amount of bound GST-Did2(CT), then plotted relative to the negative control (no GST-Did2(CT) = 0) and the maximum signal (1xIst1 = 1). (B) Survival of wild-type yeast, yeast expressing a dominant-negative version of Vps4 (vps4E233Q, pMB66), and yeast with GAL1-driven overexpression of Ist1 over a period of five days. The data show the average of three parallel experiments (+/- standard deviation). (C) Steady state localization of three permeases, Ftr1-GFP (iron), Fur4-GFP (uracil), and Can1-GFP (arginine) after extended exponential phase growth in WT and ist1Δ cells. Images were inverted for better visualization (brightest GFP signal appears black). The total and internal (excluding plasma membrane) GFP signal was quantified from 20 cells of each microscopy set. The graph shows the percentage of cells with a particular range of internal-to-total GFP signal. Analysis using the Kolmogorov-Smirnov test indicated that all discussed differences are statistically relevant.