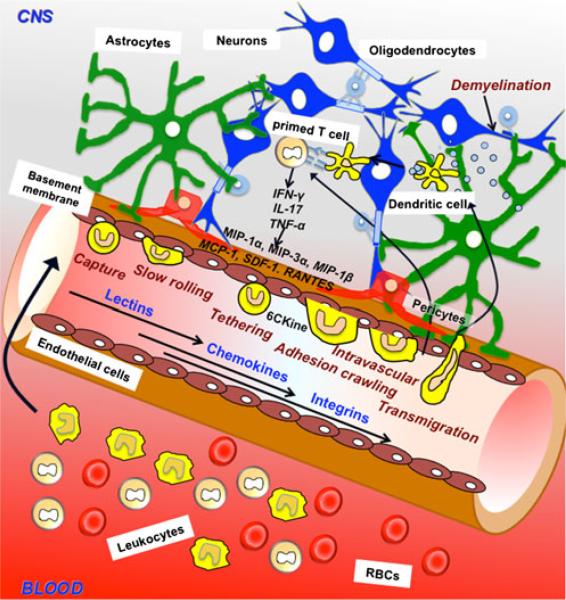
Fig. 2.
Dynamic interaction between the immune system and the blood–brain barrier (BBB). Lymphocytes are able to migrate to the central nervous system through the processes of tethering, rolling, adhesion, and transmigration through the BBB during neuroinflammation. Dendritic cells (DCs) are believed to undergo the same process but the mechanism by which the transmigration happens remains to be investigated. The circumference of the capillary lumen is completely surrounded by a single endothelial cell. Pericytes are attached to the abluminal surface of the endothelial cell, and these two cell types are surrounded by the basal lamina, which is contiguous with the plasma membranes of astrocyte end-feet and endothelial cells. Neurons also populate the area surrounding the unit. In diseases like multiple sclerosis, there is an infiltration of myelin specific T cells from systemic circulation into the CNS resulting in demyelination and increase in proinflammatory cytokines IFN-γ, TNF-α and interleukin-17 (IL-17). The components of the neurovasculature are stimulated by these cytokines and may secrete their own chemokines such as MCP-1, MIP-1, RANTES, SDF-1, CCL19, CCL20, CCL21. DCs bearing receptors for these chemokines become attracted at the vasculature and will follow the same multistep cascade as the T cells in order to transmigrate to sites of lesion. Once in the parenchymal and perivascular spaces, they may be involved in uptake and presentation of myelin peptides to T cells, that secrete more proinflammatory cytokines, thereby augmenting inflammation