Abstract
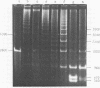
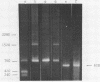
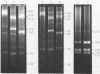
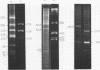
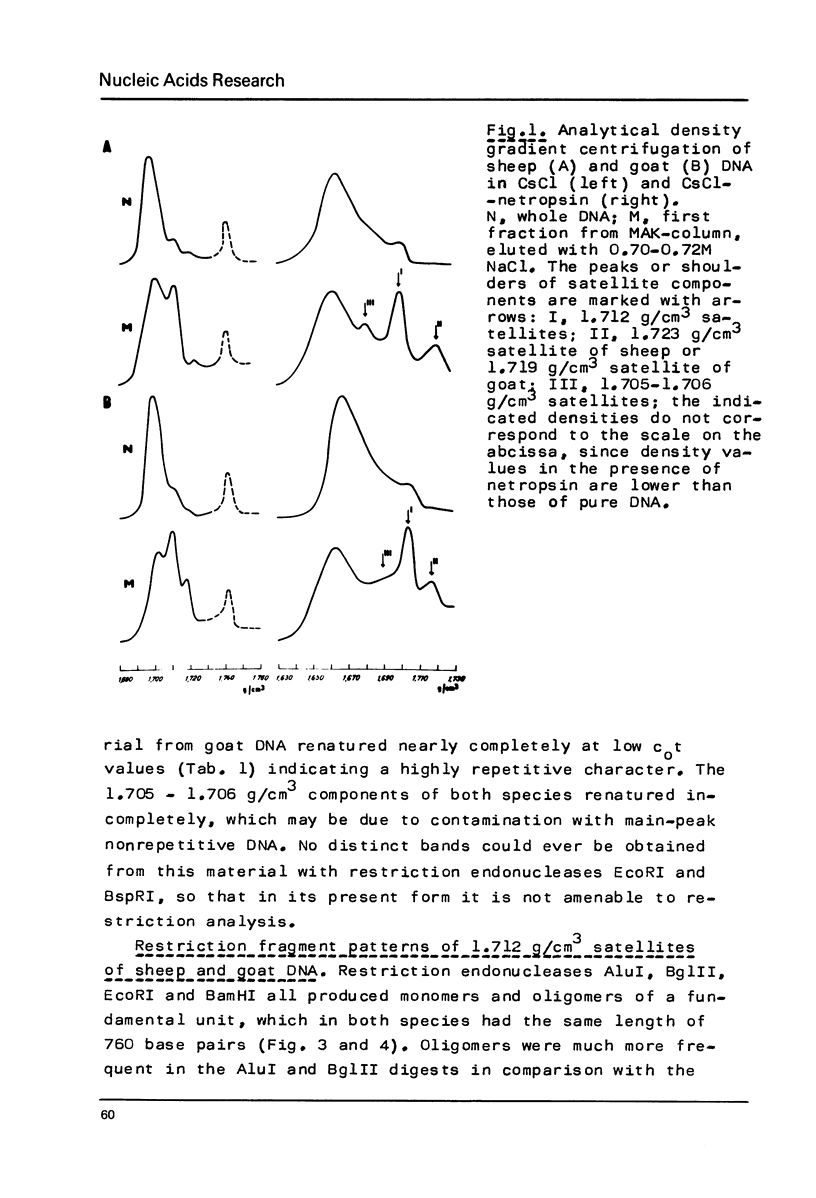
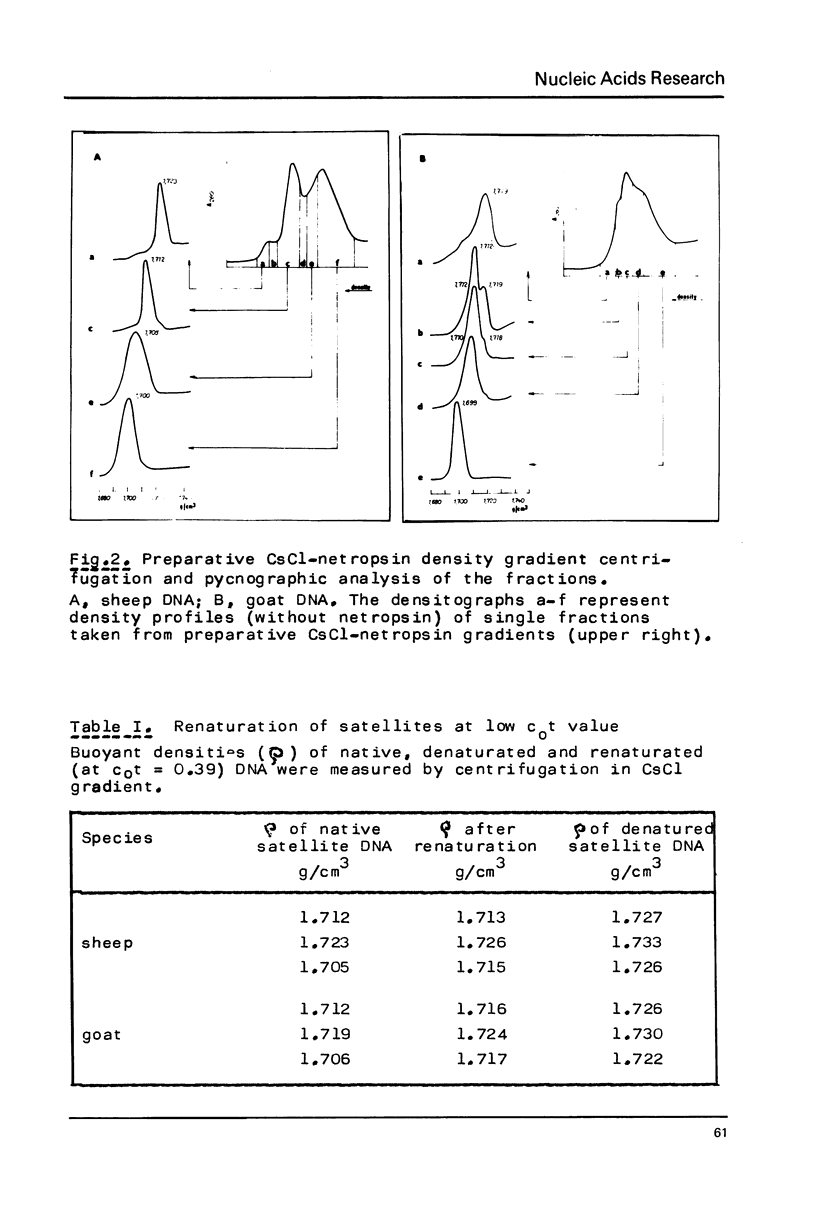
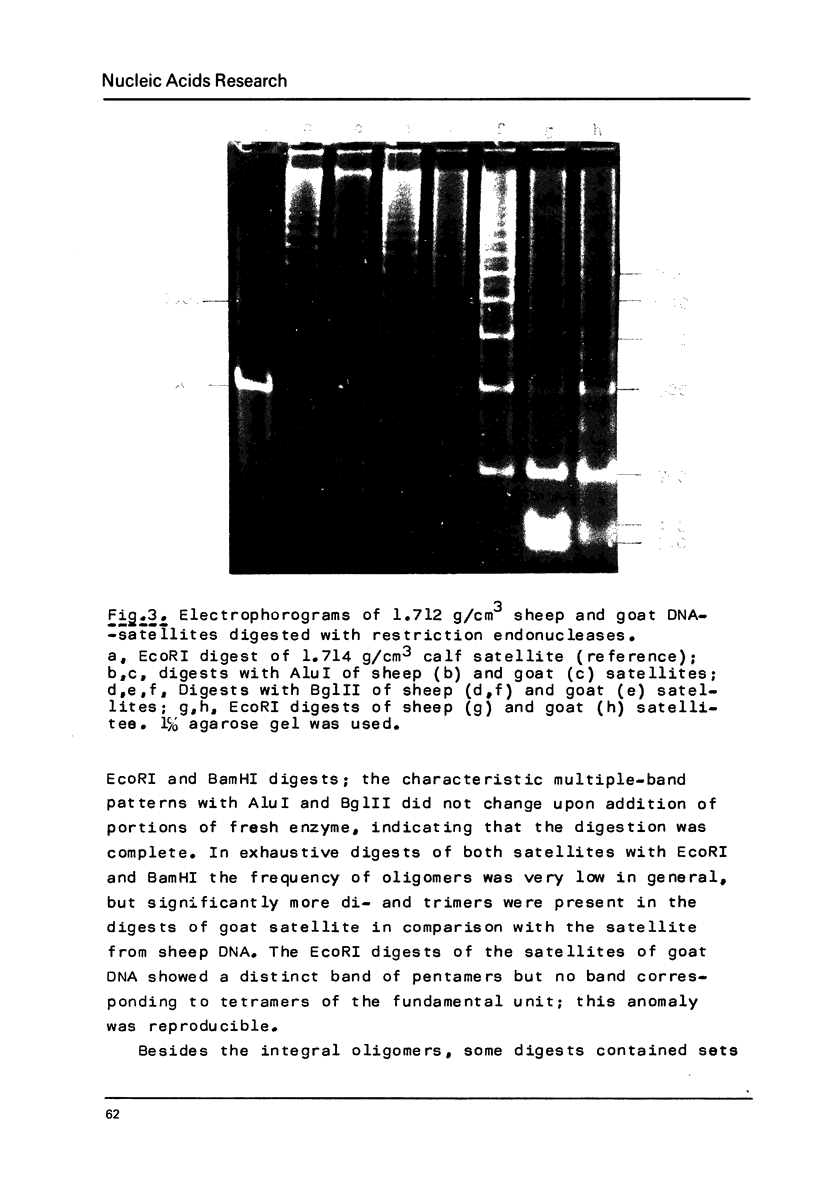
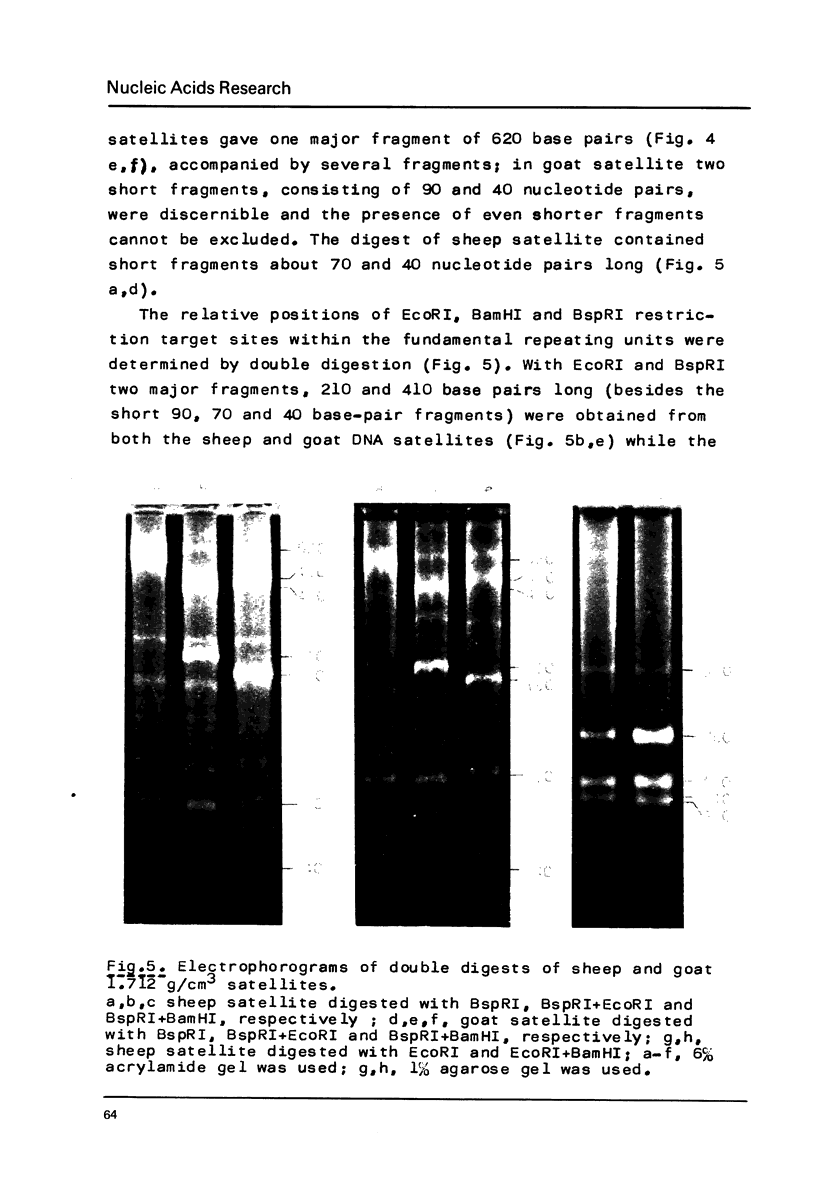
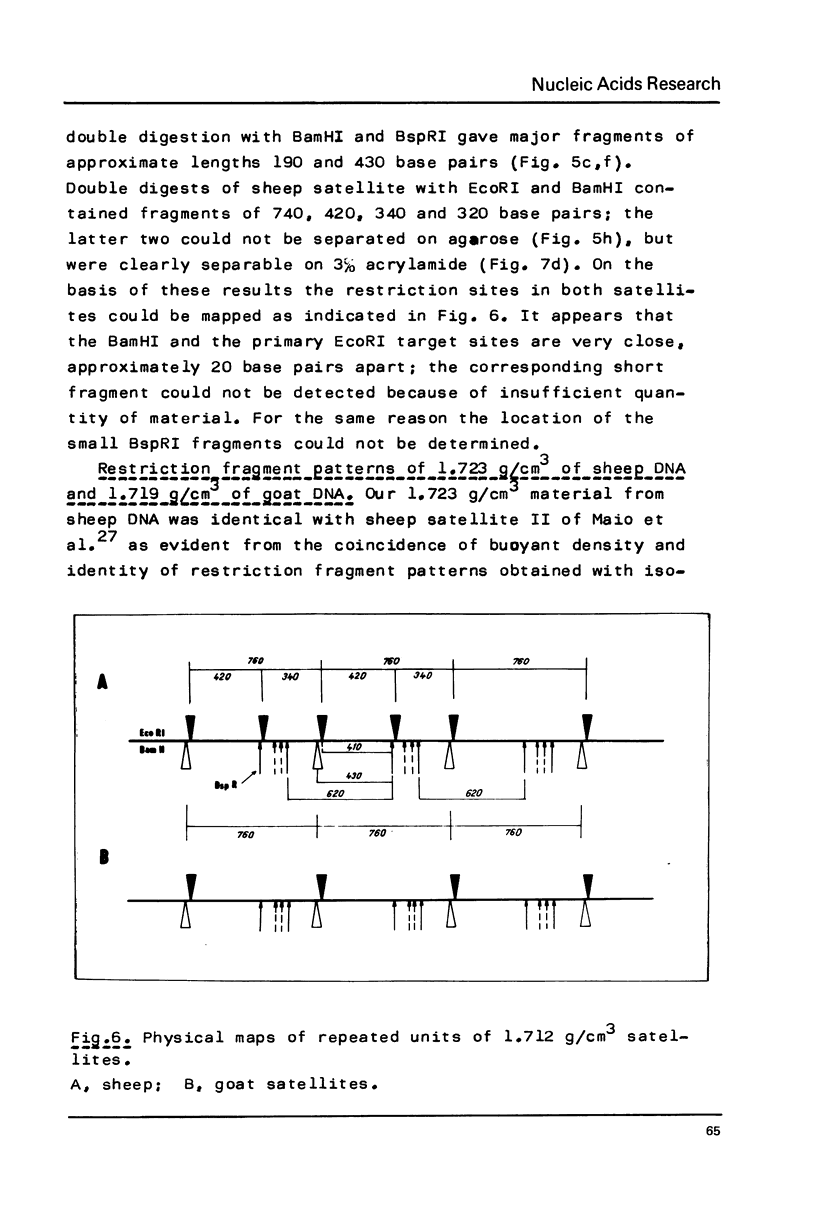
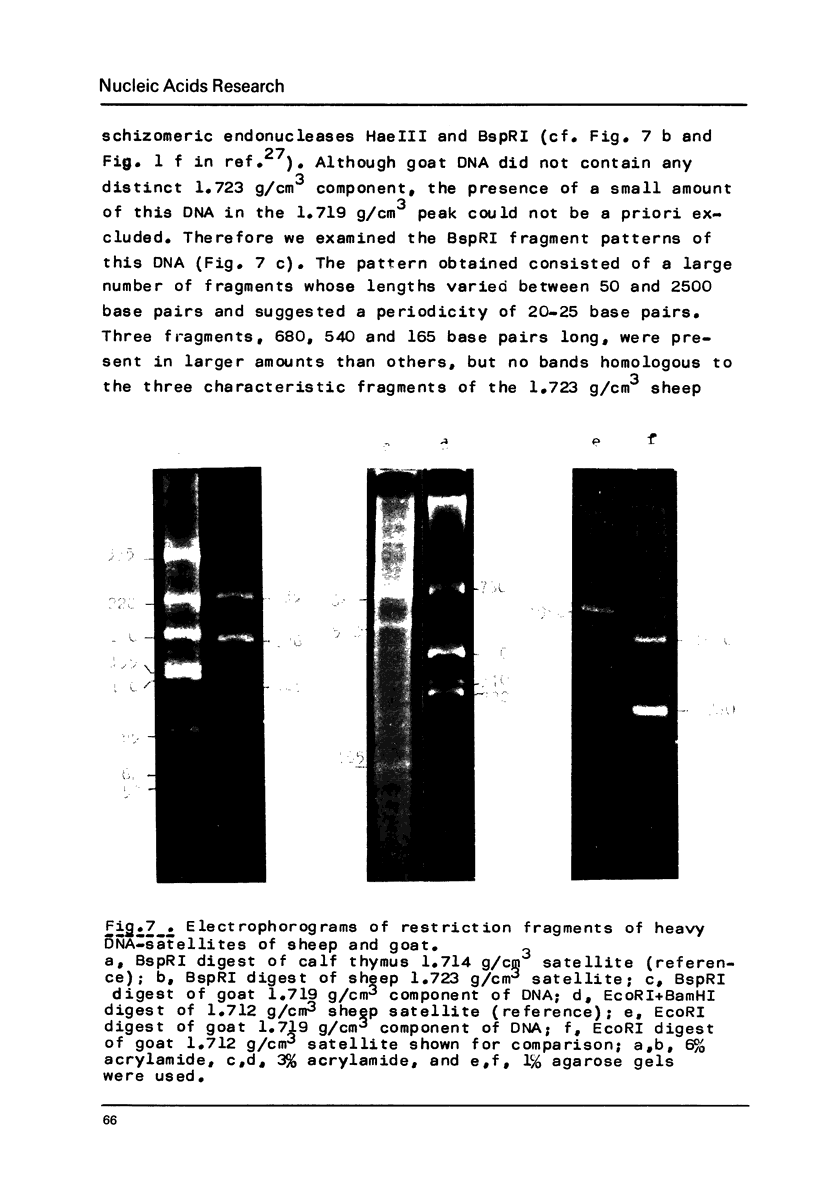
Restriction fragment patterns of G+C-rich satellites of sheep and goat DNA were compared. The 1,712 g/cm3 satellites of both species appear homologous, consisting of repeats 760 base pairs long and showing coincidence of position of primary+ EcoRI, BamHI and most BspRI restriction target sites. The EcoRI and BamHI endonucleases produce mostly monomers of the repeating unit, while oligomers prevail in the A1uI and Bg1II digests. Species-specific differences in the frequency, position and mode of distribution of secondary+ restriction target sites for EcoRI, Bg1II and A1uI were observed. Unlike the 1,712 g/cm3 satellites, the 1,723 g/cm3 component of sheep DNA and the 1,719 g/cm3 material from goat DNA appear species--specific, since no homologous material could ever be detected in the DNA of the other species.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cortadas J., Macaya G., Bernardi G. An analysis of the bovine genome by density gradient centrifugation: fractionation in Cs2SO4/3,6-bis(acetatomercurimethyl)dioxane density gradient. Eur J Biochem. 1977 Jun 1;76(1):13–19. doi: 10.1111/j.1432-1033.1977.tb11565.x. [DOI] [PubMed] [Google Scholar]
- Curtain C. C., Pascoe G., Hayman R. Satellite DNA in the sheep and goat. Biochem Genet. 1973 Nov;10(3):253–262. doi: 10.1007/BF00485703. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Langley C. H. Protein evolution and the molecular clock. Fed Proc. 1976 Aug;35(10):2092–2097. [PubMed] [Google Scholar]
- Gosden J. R., Mitchell A. R., Buckland R. A., Clayton R. P., Evans H. J. The location of four human satellite DNAs on human chromosomes. Exp Cell Res. 1975 Apr;92(1):148–158. doi: 10.1016/0014-4827(75)90648-5. [DOI] [PubMed] [Google Scholar]
- Hörz W., Hess I., Zachau H. G. Highly regular arrangement of a restriction-nuclease-sensitive site in rodent satellite DNAs. Eur J Biochem. 1974 Jun 15;45(2):501–512. doi: 10.1111/j.1432-1033.1974.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Hörz W., Zachau H. G. Characterization of distinct segments in mouse satellite DNA by restriction nucleases. Eur J Biochem. 1977 Mar 1;73(2):383–392. doi: 10.1111/j.1432-1033.1977.tb11329.x. [DOI] [PubMed] [Google Scholar]
- Kiss A., Sain B., Csordás-Tòth E., Venetianer P. A new sequence-specific endonuclease (Bsp) from Bacillus sphaericus. Gene. 1977 Jul;1(5-6):323–329. doi: 10.1016/0378-1119(77)90037-3. [DOI] [PubMed] [Google Scholar]
- Kopecka H., Macaya G., Cortadas J., Thiéry J. P., Bernardi G. Restriction enzyme analysis of satellite DNA components from the bovine genome. Eur J Biochem. 1978 Mar;84(1):189–195. doi: 10.1111/j.1432-1033.1978.tb12156.x. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Miller D. A. Evolution of primate chromosomes. Science. 1977 Dec 16;198(4322):1116–1124. doi: 10.1126/science.929190. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Streeck R. E., Zachau H. G. Investigation of the repetitive sequences in calf DNA by cleavage with restriction nucleases. Eur J Biochem. 1975 Sep 1;57(1):55–68. doi: 10.1111/j.1432-1033.1975.tb02276.x. [DOI] [PubMed] [Google Scholar]
- Pramanick D., Forstová J., Pivec L. 4 M guanidine hydrochloride applied to the isolation of DNA from different sources. FEBS Lett. 1976 Feb 1;62(1):81–84. doi: 10.1016/0014-5793(76)80021-x. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Myers P. A., Morrison A., Murray K. A specific endonuclease from Arthrobacter luteus. J Mol Biol. 1976 Mar 25;102(1):157–165. doi: 10.1016/0022-2836(76)90079-6. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Hobom G. Mapping of cleavage sites for restriction endonucleases in lambdadv plasmids. Eur J Biochem. 1975 Sep 15;57(2):595–606. doi: 10.1111/j.1432-1033.1975.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Zachau H. G. A long-range and two short-range periodicities are superimposed in the 1.706-g/cm3 satellite DNA from calf thymus. Eur J Biochem. 1978 Aug 15;89(1):267–279. doi: 10.1111/j.1432-1033.1978.tb20923.x. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- WELSH R. S. Nondegradative isolation of desoxyribonucleic acid in subunit form from calf thymus nuclei. Proc Natl Acad Sci U S A. 1962 May 15;48:887–893. doi: 10.1073/pnas.48.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. M. How different are the DNAs from related animals? Nature. 1968 Jul 20;219(5151):228–232. doi: 10.1038/219228a0. [DOI] [PubMed] [Google Scholar]
- Walker P. M. Origin of satellite DNA. Nature. 1971 Jan 29;229(5283):306–308. doi: 10.1038/229306a0. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]