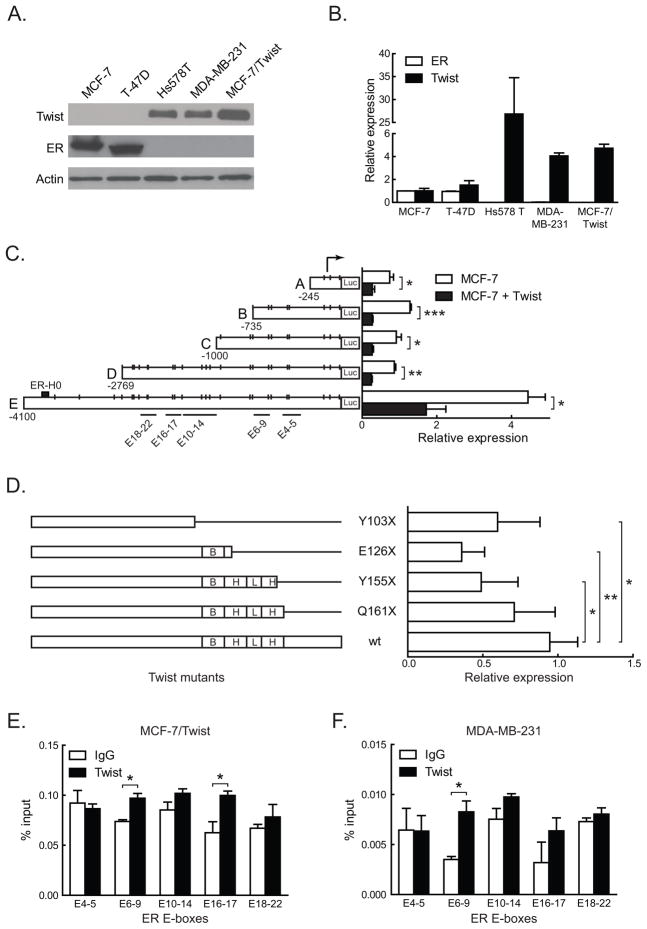
Figure 1. Twist regulation of ER by direct promoter binding.
A. Total proteins from three tumorigenic (MCF-7, T-47D, and Hs 578T) and two metastatic (MDA-MB-231 and MCF-7/Twist) breast cancer cell lines were immunoblotted for Twist and ER. Antibodies against Twist were made in-house, while ER (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (Sigma-Aldrich) were obtained commercially.
B. Histogram depicting relative expression of Twist and ER mRNA from cell lines analyzed by qRT-PCR. The primers used were 5′-GGACAAGCTGAGCAAGATTCAGA-3′ and 5′-TCTGGAGGACCTGGTAGAGGAA-3′ for Twist and 5′-GACAGGGAGCTGGTTCACAT-3′ and 5′-AGGATCTCTAGCCAGGCACA-3′ for ER. Values for Twist and ER were normalized to values in MCF-7. Experiments were repeated thrice in duplicates. Error bars depict S.D.
C. Schematic representation of ER promoter constructs showing the location of putative Twist binding E-boxes, denoted by short vertical lines. Areas spanned by chromatin immunoprecipitation (ChIP) amplicons are denoted by lines with E-box numbers. Promoter reporter assay results of transient Twist transfections in MCF-7 cells are displayed in the histogram on the right. Experiments were repeated five times in duplicates. Error bars depict S.D.
D. Schematic displaying Twist wild-type (wt) and mutant constructs. Stop codons, the DNA binding basic domain (B), and the helix-loop-helix (HLH) domain are indicated. Promoter activities of the constructs are displayed on the adjacent histogram. All luciferase activities were normalized to the activity of wild type Twist. Experiments were repeated twice in duplicates. Error bars depict S.E.M.
E–F. Chromatin immunoprecipitation using Twist antibody was carried out using MCF-7/Twist and MDA-MB-231 cells and analyzed using E-box specific primers by quantitative real-time PCR. Histograms depict the amplification from each primer set as a percentage of input chromatin compared to IgG negative control. A rabbit monoclonal antibody against histone H3 was used as positive control.